3D printing technologies such as selective laser sintering (SLM) are also spreading in the industrial small series production of complex light metal components. However, aluminum alloys such as AlSi10Mg have a higher risk of corrosion attacks in chloride-containing environments. These can be prevented by thin, active corrosion protection layers on the rough SLM surfaces. Atmospheric pressure plasma coating technology using silicone-silicate coatings with an active corrosion-protective cerium alloy is highly relevant for applications - especially for complex components. As this study shows, the time factor in particular must be taken into account when studying the chemical corrosion reactions that take place in order to achieve a high corrosion protection effect in the area of defects (scratches, pores, etc.) using low alloyed cerium concentrations.
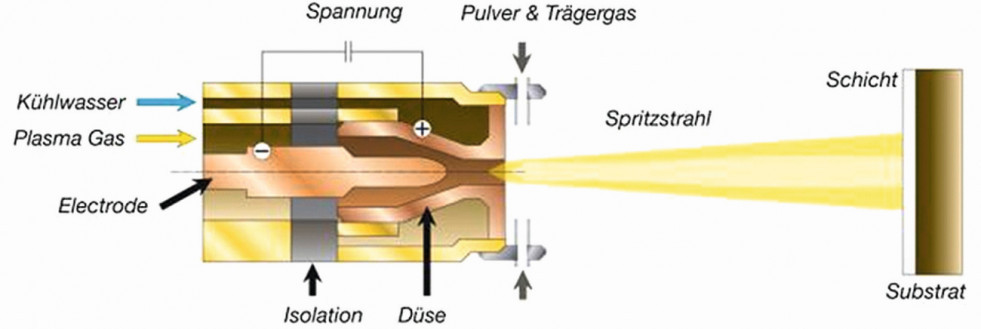 Design and operation of the patented hot gas plasma nozzle InoCoat3 from Inocon Technologie GmbH (Source: Inocon)
Design and operation of the patented hot gas plasma nozzle InoCoat3 from Inocon Technologie GmbH (Source: Inocon)
Introduction Selective laser melting of aluminum alloys
Additive manufacturing of aluminum components opens up a future growth market for European manufacturers to produce individual parts and small/medium series with complex geometries much more economically than with precision die casting or machining CNC processes (turning, milling) [9, 13, 33]. The resulting function-centred design (load, function and weight-optimized component design) opens up almost limitless design freedom in terms of shape, hierarchy, functionality and choice of material, thus saving production costs and assembly times [1, 19]. The layered structure of components allows extremely lightweight construction or resource-saving use of materials for functionally highly stressed components through targeted material utilization. Production waste (chips) is minimized compared to CNC machining, and casting moulds and dies are not required [21]. Selective laser melting (SLM) in particular is becoming increasingly established in the industrial small series production of metallic components, starting with prototype construction.
The SLM process is based on the alternating layer-by-layer application of several 10 µm thick powder layers using a doctor blade and, based on the polygonized and "sliced" data, selective laser-based melting with the underlying/adjacent layer. The disadvantages of additive manufacturing with SLM are lower mechanical properties (especially fatigue strength) due to porosity and microstructure formation. Partial sintering of powder grains occurs on the circumference of the fused areas, which, in addition to the layer waviness caused by the melt pool shape, increases the roughness and significantly reduces the cost-effectiveness of SLM due to costly reworking (sandblasting, brushing, milling of mating surfaces, etc.) [17, 43, 48].
In the field of aluminum materials, the near-eutectic alloy AlSi10Mg has established itself in SLM due to its melting behavior (low melting point, small melting interval and therefore low sensitivity to hot cracking, etc.). SLM-manufactured AlSi10Mg materials are significantly harder (Rp0.2 = 270 MPa) than cast AlSi10Mg in the initial state, but more brittle (A < 2.5%) than cast materials. Heat treatments at excessively high temperatures (solution annealing, e.g. at 550 °C) lead to a significant loss of strength with increased toughness due to structural coarsening, whereby the high strengths known for cast iron cannot be achieved even after ageing [27]. Therefore, solution annealing treatments at lower temperatures (~350-450 °C) with achievable A ~15 % at max. Rp0.2~210 MPa are preferred for SLM [7, 25, 28].
Corrosion problems of AlSi10Mg from SLM processes
This incomplete heat treatment for the necessary ductility increase leads to finely distributed noble Si particles with preferential a-Al matrix dissolution [8, 10, 35]. Since this prevents the coarser parallel-needle Si structure, which is appreciated in die casting as the basis for dense SiO2 protective layer formation during the cathodic corrosion reaction (i.e. oxygen reduction during planar ablation), especially on the surface [39], the corrosion resistance of SLM materials is significantly lower as a result. In acidic, e.g. HNO3-containing environments, this leads to corrosion rates at least 2 to 3 times higher in 0.01-1 M HNO3 [30, 39] (e.g. acidic cleaning agents) or in the salt spray test (chloride ions) to the lowest corrosion resistance class KBK 0 [21] (cf. cast AlSi10Mg: KBK 2 of 4 = medium protection). Passive protection by the Al2O3 surface formed intrinsically in air is also only present at pH values between 4.0-8.5 [39, 41], which means that alkaline cleaning agents also have a corrosive effect [21].
Local chemical and microstructural inhomogeneities in the 10-100 µm range due to the characteristic crescent-shaped molten pool formation in the installation direction additionally intensify the corrosion of SLM-AlSi10Mg [8, 26, 40]. Finally, the surface of SLM components is generally wavy due to the layered structure and rough and open-pored due to the break-out of poorly adhering particles during post-processing (shot peening) - again detrimental to corrosion [8]. Coarser porosity due to incomplete fusion ("keyhole pores") occurs increasingly at higher, economical build rates (i.e. higher laser scan speeds, higher layer thickness or track spacing) [2].
A look at advanced SLM aluminum alloys also shows increasing corrosion problems due to micro-segregation [21]: In AlSi9Cu3 with better machinability due to lower Si content, occurring Al2Cuand AlCu2Mgprecipitations locally enhance the cathodic reaction and oxygen reduction. AlMgSc (Scalmalloy), which is particularly important in the aerospace sector and has twice the yield strength of AlSi10Mg, is highly susceptible to pitting due to Al8Mg2 or Al3Mg2.
Corrosion protection of aluminum alloys
To improve the corrosion resistance of components made of aluminium alloys, either anodizing treatments or conversion coatings are currently used as a state of the art technique (regardless of the manufacturing process used) after chemical cleaning or pickling pre-treatment in HNO3+H3PO4 to remove oxide layers and precipitates on the surface. However, the disadvantages of anodizing are the need for chemical baths and the increased visibility of material defects. Optically decorative parts therefore require a final coating with loss of hardness and wear/abrasion resistance. Conversion coatings (e.g. chromating) generally offer active corrosion protection even at the very low thicknesses used. Wet-chemical application is usually carried out in baths containing chromic acid, although the use of the optimal protective but carcinogenic chromium(VI)-based starting baths [11] is severely restricted by law due to the EU End-of-Life Vehicles Regulation, RoHS and REACH. Chromium(VI)-free (i.e. without chromate Cr2O72-), non-carcinogenic process management, on the other hand, severely restricts the achievable corrosion protection effect, as self-healing is made more difficult by the formation of an Al/Cr3+ hydroxide on contact with water. Chromate was also used as an inhibitor in pigments (as a salt with Ca, Sr, Ba and Zn cations) in primer paints with a similar mode of action. Other less widely used corrosion protection methods for aluminum are electroplating, painting, sol-gel, PVD, CVD and thermal spray processes [8].
As described above, the combination of wear and corrosion protection of aluminum often poses major problems for state-of-the-art technology. Plasma coatings are an alternative, although high vacuum processes (~1 Pa) for physical or (plasma-assisted) chemical vapor deposition (PVD, (PA-)CVD) are hardly economically viable. The WICOATEC process for CVD coating of heat exchangers at > 300 °C in a fine vacuum (~100 Pa) [46] has already been implemented in industry, which also allows uniform coating on components with a high aspect ratio.
Cheaper alternatives to high and fine vacuum are atmospheric pressure plasma processes, which do not require a cost-intensive reaction vessel (vacuum chamber):
Plasmas for coating purposes can be technically generated by nozzles (plasma jet) or dielectric barrier discharge (DBD). With plasma jets, a high-frequency ignition pulse (10 kV) is used to generate an arc and maintain it at a constant current under voltage control, through which the process gas flows and is ionized (see illustration on p. 1270). The outlet is point-shaped through a nozzle head as a thermal hot gas plasma, which is at ground potential and thus largely retains potential-carrying parts of the plasma flow. In particular, the internal structure of the plasma nozzle and the excitation voltage/frequency used define the plasma properties that can be achieved (density, energy, etc.).
Atmospheric plasmas have been used for many years, particularly in the field of plastics pre-treatment and adhesion promotion. For some years now, commercially available free jet plasma systems (e.g. from PlasmaTreat or Inocon Technologie GmbH) have also been available for plasma pre-treatment, but also for the deposition of thin functional coatings. However, their use in the field of "Atmospheric Pressure Plasma Chemical Vapor Deposition" (APCVD) is still in its infancy and focuses on application-based coating development [5, 6, 14, 22, 37, 38, 42, 50]. The layer formation can take place (1) starting from organic precursors (e.g. HMDSO, TEOS, TiCl4, C2H2, etc.) via radical formation as a result of collision with surrounding particles and the very intensive UV radiation in the plasma [16] (plasma polymerization) [48]. The precursors can be added in the highly ionized discharge zone or only in the zone of quasi-neutral plasma ("afterglow") without interaction of electrons and ions, whereby in the former case the forming polymer structure is decisively damaged by the ionization of the precursor [32]. Salts can be introduced into the plasma via precursors or alcoholic solutions and used to modify the layer. The use of aerosols protects the polymer structure in the droplets from plasma exposure [14, 18, 20], but results in the formation of rough, porous layers with/from micro-droplets (depending on the vapor pressure) [34]. (2) Alternatively, or in combination [4, 29, 45] with precursors/aerosols, metallic or ceramic micro/nano particles can also be added to the plasma at the nozzle outlet, whereby these are then melted or vaporized in the plasma depending on their size and deposited as a layer on the substrate, possibly after superficial oxidation or coating with the precursor ("plasma spray"-like process) [12]. The general advantages of APCVD coatings over high/fine vacuum coatings are the high deposition rates (1-10 µm/s*cm2) as well as the elimination of expensive vacuum vessels. The low porosity (< 0.1 %) and the high coating thickness (> 10 µm) that can be achieved without cracking (due to the possibility of multiple applications without time intervals) as the basis for high passive corrosion protection, the high coating adhesion (chemical bonding) and especially the excellent wear properties (e.g. of silicate coatings) are advantageous compared to sol-gel, lacquer and electroplating coatings.
Corrosion inhibitors and their use in coatings for aluminum
Corrosion inhibitors generally reduce the corrosion rate: (1) Anodic inhibitors such as toxic chromates (Cr(VI)) reduce the anodic reactions by reacting with the corrosion product (metal ion) and forming a well-adhering, insoluble protective passivation layer on the metal surface. Sufficient concentration is important to prevent localized pitting corrosion. Alternatively applicable molybdates [49] are only effective under moderate alkaline conditions. Silicate inhibitors have their mechanism of action in the formation of a hydrated metal-silicate layer on the active surface, whereby organically modified silicate layers (Ormosil®) are of greatest importance [24], but are limited to < 1 µm layer thickness due to residual stresses occurring during formation (hydrolysis) from solutions. Compounds with rare earths, i.e. cerium, yttrium and praseodymium, also offer good anodic inhibitors for Al alloys. A cerium-rich conversion layer is formed from CeCl3 in particular. If OH is formed during corrosion, this reacts with Ce3+ to form insoluble, well-adhering, corrosion-inhibiting CeO2. The combination of these elements generally increases their effect.
Since HMDSO layers provide passive barrier protection, this is very promising in combination with an active system that can repassivate initial corrosion attack sites. If there is a sufficient concentration of cerium ions in the coating, a cerium hydroxide layer can form at small defects (pitting sites) by leaching. This poorly soluble hydroxide layer is deposited over the defect and thus leads to "self-healing" [31].
Cathodic reaction, formation of insoluble hydroxides: Alternatively, Ce3+ can be oxidized to Ce4+ by peroxide:
(2) Cathodic inhibitors prevent the cathodic reaction by the formation of e.g. insoluble hydroxides (e.g. Mg(OH)2, Zn(OH)2) on the cathodically acting surfaces. Polyphosphates, phosphonates, tannins, lignins and calcium salts also have a similar inhibitor mechanism. Anodic, cathodic and mixed inhibitors can also be realized with organic compounds, but the achievable effect is significantly lower [47].
The use of corrosion inhibitors - as particles and as salts homogeneously distributed in the matrix - in mostly organosilicon APCVD coatings (APCVD) has only been described scientifically in very few cases and is not yet in commercial use despite its potential. In this context, the work of the Tudor Institute [3, 4, 15, 16] is decisive. In silicone-like layers, SiO2 nanoparticles lead to improved passivation of aluminum by reaction with OH- and Al3+ ions [16], which is further enhanced by CeO2-SiO2 nanoparticle composites due to the O2 diffusion barrier when CeO2 accumulates in pores and near the substrate surface and, at high concentrations (> 5 %), also enables self-healing in the event of pitting. Similar to the use of AlCeO3 nanoparticles [4], the required Ce concentrations are at least 2 % [16], whereby homogeneous dispersion in the APCVD plasma, e.g. by ethanol, is of particular importance [4].
Experimental
The aim of the work was to produce active corrosion-protective coatings based on organosilicon precursors (hexamethyldisiloxane) and cerium salts using atmospheric pressure plasma coating technology under industrially relevant conditions, i.e. component geometries, technical surface qualities, coating rates, etc.. The plasma jet used was an IC3 from Inocon Technologie GmbH (Attnang-Puchheim, Austria), which was initially used for cleaning and activating the substrates to increase the adhesive strength using argon plasma, and then for coating deposition with simultaneous application of HMDSO as an evaporated precursor and aqueous cerium salt solution with air as the carrier gas (and partial oxygen for the reaction to form silicate). An illustration of the plasma jet, including the resulting plasma, is shown on page 1270. The cerium salt solution, i.e. Ce(III) nitrate (Ce(NO3)3 - 6 H2O) as a 0, 10, 12.5 and 15 % aqueous solution, was mixed as an aerosol (generated in the Topas ATM 2010 aerosol generator, Dresden, Germany) into the discharge plasma (170 A discharge current). 0 % salt in the aerosol was added as a parameter for the coatings in order to take into account the energy required for the evaporation of the water as an aerosol base and its influence on the deposition of silicon organic coatings. The relative speed between the substrate and the plasma jet in this "aerosol-assisted atmospheric pressure plasma deposition" process was 100 mm/s, with a distance of 50 mm. Due to the narrow expansion of the atmospheric pressure plasma, the surface of the samples was scanned, i.e. passed over twice alternately in the x and y directions with a track spacing of 5 mm. In order to investigate the corrosion protection effect, aluminum plates were used as AlSi1MgMn sheets (EN-AW 6082) with a ground surface and as SLM-printed AlSi10Mg (EN-AB 43400) with a blasted standard surface after SLM production (each with a size of 40x40 mm2 and a thickness of 1 mm).
The application-related corrosion tests were carried out using an Erichsen "Corrocompact 616" salt spray chamber in accordance with DIN EN ISO 9227. Before the test, the edges of all coated samples or parts of the demonstrator components were sealed with a primer to prevent material attack at the interfaces. A solution of sodium chloride and deionized water (50 g/L) was used as the corrosive medium, which was adjusted to a pH value of 7 ± 0.2 with HCl or NaOH. Approximately 0.4 l/h of the solution was evenly distributed in the chamber through the spray nozzle. For better wetting of the samples, they were positioned at an angle of 20° to the vertical. The samples were documented by photographs in their initial state, after 24, 96, 168, 264, 360, 432, 600 and 768 hours. In order not to falsify the stress over the entire duration of the test, the samples were not cleaned before documentation.
Detailed scientific studies on corrosion behavior were carried out using potentiostatic and potentiodynamic measurements in physiological saline solution (0.9 % NaCl) at 25 °C (VersaSTAT 4 Potentiostat, Galvanostat with VersaStudio software V2.42.3). The surface area of the coatings examined was always 1 cm2. The sample, the platinum electrode and the saturated calomel electrode (SCE) are used as working, counter and reference electrodes respectively.
Immersion tests were performed in SBF solution with 0.9 % NaCl (isotonic aqueous solution, PF % 0.9 Izotonik, Polifarma) at 37 ± 1 °C for 1, 6, 24, 48, 72 and 96 hours. After each immersion time, the electrolyte was analyzed with ICP-MS (inductively coupled plasma mass spectrometer, Perkin Elmer Elan DRC-e). The ICP-MS system used is equipped with a Cetax ADX-500 auto-sampler and diluter to qualify and quantify the released elements.
Profilometry (Veeco Dektak 150) was used to measure layer thickness and roughness. Scanning electron microscopy (Tescan Vega 3, Jeol 6060) with energy dispersive X-ray spectroscopy (EDX) for elemental analysis (OxfordULTIM MAX 40, I-XRF) was used to investigate the surface topography. X-ray photoelectron spectroscopy (XPS, Omicron Multiprobe system) was used to determine the cerium content as accurately as possible with a detection sensitivity of 1 mass % and Ar+ ion sputtering to exclude oxidation effects on the surface.
Results and discussion
In XPS analyses, cerium could be detected in the organosilicon coatings in the range of 0.1 to 0.4 at.%, whereby the concentration is similar to that described in the literature as corrosion-protective [23, 31, 44]. In connection with the solubility limit of cerium(III) nitrate in water (aerosol starting solution, ~15 mass%), solutions with 10 to 15 mass% cerium were used for the subsequent investigations.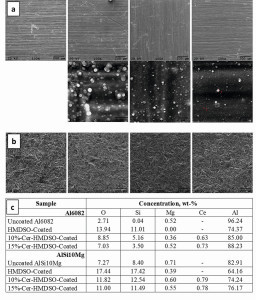 Fig. 1: SEM images of (from left to right) uncoated and from HMDSO precursor SiOx-coated aluminum surfaces with 0, 10 and 15 % cerium content for (a) AlSi1MgMn sheet (Al6082) and (b) SLM-AlSi10Mg. (c) EDX surface analysis of the above coatings on both substrate types (All illustrations, photos, diagrams: Joanneum Research)
Fig. 1: SEM images of (from left to right) uncoated and from HMDSO precursor SiOx-coated aluminum surfaces with 0, 10 and 15 % cerium content for (a) AlSi1MgMn sheet (Al6082) and (b) SLM-AlSi10Mg. (c) EDX surface analysis of the above coatings on both substrate types (All illustrations, photos, diagrams: Joanneum Research)
In the SEM images of coated aluminum surfaces shown below, different surface topographies are visible (Fig. 1), i.e. small spherical structures, especially in the high magnification images of cerium-containing coatings, while they are hardly visible at low magnification due to the high roughness. These fine structures are independent of the alloy of the substrate and its production-related roughness. In general, numerous scratches and defects occur. In the AlSi1MgMn sheets, these are grinding grooves with unidirectional alignment, while in the SLM AlSi10Mg samples, sintered structures leveled by sandblasting with barely recognizable directionality. The roughness itself is around 5-8 µm on the ground sheet (depending on the direction) and around 10-12 µm on the SLM samples. Due to their great depth, even the coatings applied cannot level out these substrate defects, as the layer thickness of pure HMDSO layers on Al is ~2200 nm and of HMDSO layers with 10 % and 15 % cerium only ~900 nm. These scratches and defects on the coating surfaces are undesirable with respect to corrosion and may represent the initiation pathways for the corrosion process. The study design using these substrates therefore reflects near-application conditions for corrosion protection and the active corrosion protection mechanisms and thus self-healing effects through the protective mechanisms of cerium.
The EDX analysis of the surface in Fig. 1c shows a weight ratio of Si/O ~ 1, indicating a SiO2-like coating (molecular weight of Si = 28.08 g/mol, O = 15.99 g/mol). The cerium content is also easily recognizable, but somewhat overestimated due to the significantly lower accuracy of EDX compared to XPS.
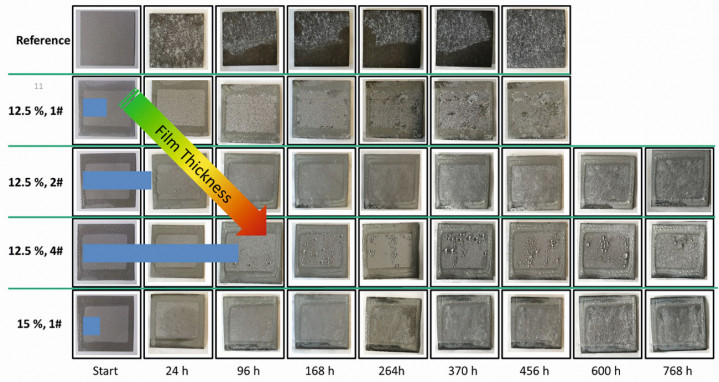 Fig. 2: Salt spray test on SLM-AlSi10Mg samples (12.5 % and 15 % cerium as cerium(III) nitrate in the aerosol)
Fig. 2: Salt spray test on SLM-AlSi10Mg samples (12.5 % and 15 % cerium as cerium(III) nitrate in the aerosol)
The results of the salt spray test in Figure 2 show that the coating thickness (~300, 700 and 1500 nm for the aerosols with 12.5 % cerium nitrate in water (samples 1#, 2#, 3#)) plays a decisive role in the formation of corrosion, irrespective of the cerium content, and that there is therefore a correlation with surface roughness. In the uncoated SLM-AlSi10Mg samples, salts from the electrolyte are already deposited after 24 h - which is associated with underlying attack. Individual areas are already dark and heavily corroded after 96 hours. These signs of corrosion are not visible in the coated samples. Corrosion only occurs very locally here. SEM examinations (Fig. 3) confirm that, in contrast to the reference, no pitting occurs on the smoother, coated Al sheets. On the reference, this is recognizable as a black dot with increased oxygen content (EDX analysis) (30-40 at% higher than in the surrounding bright areas). On all rough SLM-AlSi10Mg substrates, pitting can be found in places, which is accompanied by an increased oxygen content (40-50 at% higher than on the unaffected areas). Nevertheless, the coatings also offer very good protection here, if the samples are compared with the more clearly corroded uncoated substrates. This also correlates with the higher salt coverage. Droplets are easily recognizable on the hydrophilic surfaces post-oxidized in the oxygen plasma. Further results not shown here indicate that post-oxidation has no significant influence on corrosion, although the oxidation of the coating surface can increase the water surface energy to ~65 mN/m (hydrophilic), while this is around 20-30 mN/m for non-post-oxidized coatings (hydrophobic, lower with higher cerium content. Comparison with Al substrate: ~56 mN/m). Since the drops of the test aerosol roll off better on hydrophobic, more silicone-like coatings in the salt spray test, these are better suited for splash protection and self-cleaning.
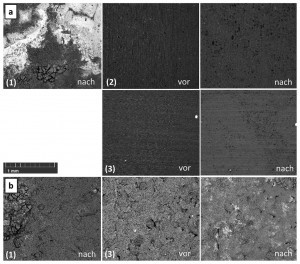 Fig. 3: SEM evaluation of samples made of (a) AlSi1MgMn sheet and (b) SLM-AlSi10Mg before and after the salt spray test: (1) uncoated reference, (2) cerium-free HMDSO-SiOx coating (without aerosol), (3) HMDSO-SiOx coating with aerosol with 15 % cerium nitrate content
Fig. 3: SEM evaluation of samples made of (a) AlSi1MgMn sheet and (b) SLM-AlSi10Mg before and after the salt spray test: (1) uncoated reference, (2) cerium-free HMDSO-SiOx coating (without aerosol), (3) HMDSO-SiOx coating with aerosol with 15 % cerium nitrate content
Detailed scientific studies on the corrosion behavior were carried out with potentiodynamic, potentiostatic and impedance spectroscopy measurements in non-deaerated 0.9% NaCl electrolytes with a three-electrode cell. The potentiodynamic polarization curves of AlSi1MgMn sheet and SLM-AlSi10Mg are shown in Figure 4. The corrosion parameters such as the corrosion potentialEcor, the corrosion currenticor and the polarization resistance Rp, which were determined from these curves, are also shown in Figure 7.
The analysis of the data shows that on the smoother AlSi1MgMn surface, the corrosion currents of the cerium-free layer in the diagrams are the lowest, followed by the cerium-containing layer of 15 % cerium nitrate-containing aerosol. For the rougher SLM-AlSi10Mg samples, on the other hand, the corrosion currents are generally higher, but lowest for SiOx layers with cerium. The sheet resistance Rp generally increases with cerium content, as does the inhibition efficiency IE% with high cerium contents to at least 50 % higher values than with cerium-free coatings, although the latter are about twice as thick.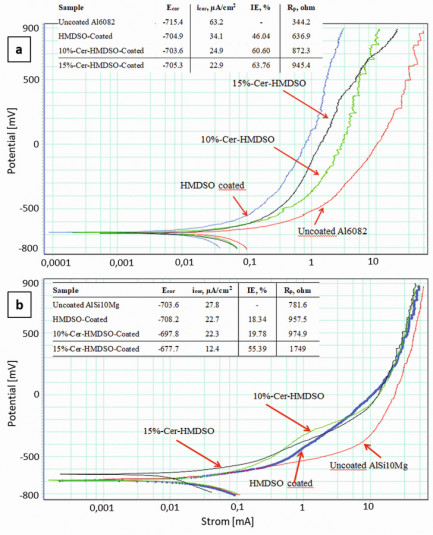 Fig. 4: Potentiodynamic polarization curves of uncoated or HMDSO without and with 10 and 15 % cerium in the aerosol coated (a) AlSi1MgMn sheet (Al6082) and (b) SLM-AlSi[[[Mg. Calculation results for corrosion potential (Ecorr), corrosion current (icorr) and polarization resistance (Rp) are also given
Fig. 4: Potentiodynamic polarization curves of uncoated or HMDSO without and with 10 and 15 % cerium in the aerosol coated (a) AlSi1MgMn sheet (Al6082) and (b) SLM-AlSi[[[Mg. Calculation results for corrosion potential (Ecorr), corrosion current (icorr) and polarization resistance (Rp) are also given
Corrosion currents were also recorded at constant corrosion potential (+0.1, +0.5 and +1 V) (potentiostatic measurement after 10 min exposure in physiological saline solution), whereby samples of SLM-AlSi10Mg with high roughness (as-print, Ra ~12 µm) and after standard sandblasting after SLM printing (Ra ~5 µm) before coating are shown in Figure 5. The uncoated reference samples show the expected reduced corrosion currents (and thus lower corrosion rates) with smoother, blasted surfaces. As can also be seen from the above polarization curves, the coated samples exhibit significantly lower corrosion currents, which are, however, hardly influenced by the cerium content, especially on the smoother sandblasted surfaces. The rough, as-built samples show clearer differences towards lower corrosion currents with a higher cerium content compared to cerium-free HMDSO layers. It is also interesting to note that thinner coatings without cerium content (aerosol-assisted deposition with cerium-nitrate-free water) reduce the corrosion currents more than cerium-containing coatings. This could be related to changes in the layer formation (lower energy in the plasma, changed layer structure with higher residual organic content, lower residual layer stresses, etc.).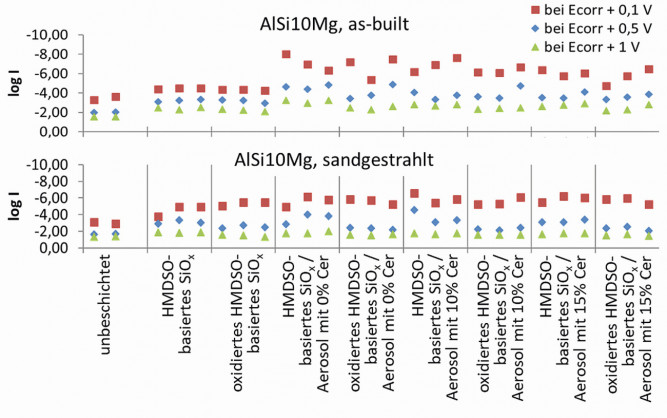 Fig. 5: Dependence of the corrosion currents (and thus current densities) of as-built and sandblasted SLM-AlSi10Mg samples in the potentiostatic test setup with specified corrosion potentials Ecorr of +0.1, +0.5 and +1V
Fig. 5: Dependence of the corrosion currents (and thus current densities) of as-built and sandblasted SLM-AlSi10Mg samples in the potentiostatic test setup with specified corrosion potentials Ecorr of +0.1, +0.5 and +1V
Potentiodynamic polarization measurements (PDP) show that the coated samples only corrode at higher potentials in 0.9 % physiological saline solution compared to the uncoated samples. In general, it was found that the tendency to passivation (after short-term exposure) is greater for the rough SLM-AlSi10Mg samples. The smoother (sandblasted) samples only passivate at more positive potentials. Figure 6 shows the differences between the various coating systems: Corrosion, passivation and pitting corrosion potentials are lowest for the cerium-free coating systems, but the SiOx coating produced with 15 % cerium nitrate in the aerosol is also significantly lower than the values of the uncoated samples. The current densities occurring are highest for the SiOx layers without cerium and lowest for the aluminum surfaces without coating, which apparently show self-passivation.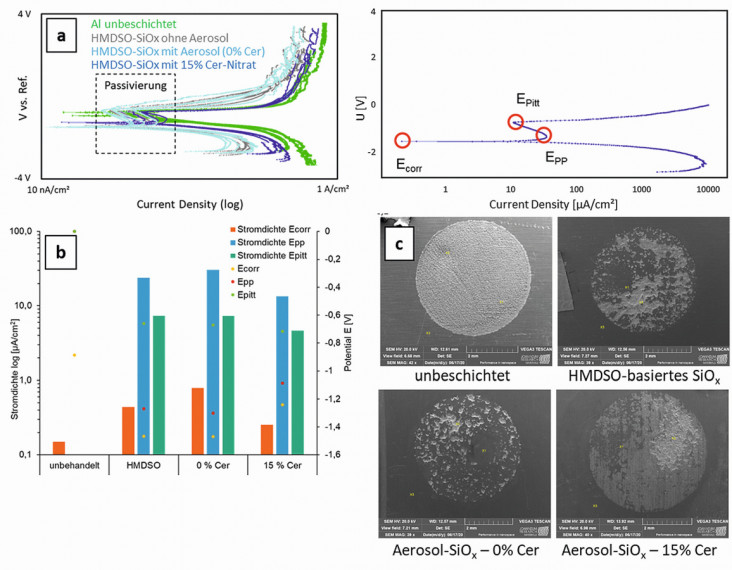 Fig. 6: Potentiodynamic measurements on HMDSO-SiOx layers without and with aerosol addition (0 and 15 % cerium nitrate in the aerosol) on SLM-AlSi10Mg: (a) Current density-potential curves and designation of the points important for passivation. (b) Corrosion potential (Ecorr), passivation potential (Epp), pitting corrosion potential (Epit) and the resulting current densities, (c) SEM images of the samples after the potentiodynamic measurement
Fig. 6: Potentiodynamic measurements on HMDSO-SiOx layers without and with aerosol addition (0 and 15 % cerium nitrate in the aerosol) on SLM-AlSi10Mg: (a) Current density-potential curves and designation of the points important for passivation. (b) Corrosion potential (Ecorr), passivation potential (Epp), pitting corrosion potential (Epit) and the resulting current densities, (c) SEM images of the samples after the potentiodynamic measurement
In general, the corrosion of aluminum alloys in aerated NaCl solution proceeds according to the reaction shown below:
O2 + 2H2O+ 4e- → 4 OH- (cathodic reaction)
Al → Al+3 + 3e- (anodic reaction)
The presence of cerium ions in the solution leads to the formation of a mixed film of CeO2, Ce(OH)4 and Ce(OH)3 according to the reactions described below:
O2 + 2H2O+ 4e- → 4 OH- (cathodic reaction)
Ce+3 + 3OH- → Ce(OH)3 (anodic reaction)
On the other hand, cerium is present in the dissolved state as Ce+3 and Ce+4. The corrosion of both ions can be estimated based on their affinity for oxygen and the standard free energy for the formation of oxides. The effect of cerium as a corrosion inhibitor depends on the oxidation state. Ce+3 has a greater potential as a corrosion inhibitor than Ce+4 (these energies for Ce2O3 and CeO2 are 411.5 and 230.0 cal mol-1 respectively). Corrosion inhibition occurs in two steps: (1) insoluble oxide/hydroxide of Ce+3 is formed by the reaction with hydroxide ions at the cathodic sites and (2) the formed insoluble Ce-hydroxide/oxide compound precipitates on the surface. However, these two steps require more than 24 hours to act as corrosion inhibitors - i.e. they are well accessible in salt spray tests with long test durations, but not in short-term electrochemical measurements. It was also found that for effective corrosion protection, the minimum concentration of Ce+3 should be around 5 ppm in neutral NaCl solution. The presence of Ce+3 in the solution plays a key role in the protection of aluminum.
Therefore, the next step was to carry out aging experiments by recording EIS spectra of 10 %-Cer-HMDSO and 15 %-Cer-HMDSO at different time intervals. Similar to [36], it was found that at lower cerium concentrations (10 % aerosol) a high protective effect occurs after 24 h due to the active formation of Ce oxides, while at higher cerium concentrations (15 % aerosol) there is also a - significantly greater - increase in polarization resistances, but no maximum was achieved within the test duration of 96 h.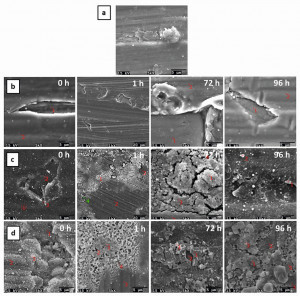 Fig. 6: Potentiodynamic measurements on HMDSO-SiOx coatings without and with the addition of aerosol (0 and 15 % cerium nitrate in the aerosol) on SLM-AlSi10Mg: (a) Current density-potential curves and designation of the points important for passivation. (b) Corrosion potential (Ecorr), passivation potential (Epp), pitting corrosion potential (Epit) and the resulting current densities, (c) SEM images of the samples after the potentiodynamic measurement
Fig. 6: Potentiodynamic measurements on HMDSO-SiOx coatings without and with the addition of aerosol (0 and 15 % cerium nitrate in the aerosol) on SLM-AlSi10Mg: (a) Current density-potential curves and designation of the points important for passivation. (b) Corrosion potential (Ecorr), passivation potential (Epp), pitting corrosion potential (Epit) and the resulting current densities, (c) SEM images of the samples after the potentiodynamic measurement
The surface analysis using SEM/EDX of uncoated and coated AlSi1MgMn sheet before and after the immersion test at different immersion time intervals is shown in Figure 7. The focus of the investigation is on defects that generally trigger corrosion if they are already present prior to aging. The use of cerium as active corrosion protection in the coating shows that, compared to purely passive HMDSO-SiOx corrosion protection, deposits are always formed that have a structure typical of salt or amorphous oxides. Additional information is provided by EDX analysis within the defects, on the deposits and on the undamaged coating surface. The purely HMDSO-coated surface shows higher oxygen contents than the areas of the defect. Compared to the uncoated sample, this is due to the base material (AlSi1MgMn) and the relatively high penetration depth of the electron "club" in the SEM analysis. For the latter reason, the cerium-containing layers, which are less than half as thick, also have a lower oxygen content and higher Al content. Defects with the layer exposed down to the Al surface are absent in the cerium-containing layers in the samples after aging. The previously existing defects are covered by deposition due to the active corrosion protection effect of Ce (salt formation). The cerium content is also greatly increased in these defects, as is the oxygen content. The Si content is also higher, the Al content lower. There are no changes in the Si content in the defect areas of the cerium-free layers, although an active corrosion protection effect is often discussed for SiOx. The findings from the above (applied) scientific studies were applied to the coating of SLM-printed demonstrators (alloy: AlSi10Mg), which contain strong 3D topography and macroscopic surface structures (honeycomb structure) and reproduce real components. Coating was done with HMDSO and aerosol additive, i.e. 1 type without cerium (pure water in the aerosol) and 1 type with cerium-loaded aerosol (10 % cerium as cerium(III) nitrate). The results of the salt spray test of the handlebar supports are shown in Figure 8. The protective effect of the coatings is clearly visible: There are significantly fewer salt deposits and corrosion spots than on the uncoated reference demonstrators made of AlSi10Mg. These could not be completely removed even with ultrasonic and mechanical cleaning with a cleaning sponge. Removed salt residues showed very dark, black surfaces, which are typical for pitting corrosion. In contrast, the coatings in all areas of the samples were able to retain the metallic gloss of the samples without spot corrosion. Fig. 8: Salt spray test on SLM-printed demonstrators made of AlSi10Mg with detailed analysis of the surfaces after768 h (from left to right: 3D CAD model, uncoated demonstrator coated with pure HMDSO-SiOx or demonstrator coated with SiOx and aerosol with 10 % cerium nitrate)
Fig. 8: Salt spray test on SLM-printed demonstrators made of AlSi10Mg with detailed analysis of the surfaces after768 h (from left to right: 3D CAD model, uncoated demonstrator coated with pure HMDSO-SiOx or demonstrator coated with SiOx and aerosol with 10 % cerium nitrate)
Summary
The present work on smooth sheet metal samples made of corrosion-prone aluminum alloy (AlSi1MgMn) as well as the significantly rougher SLM-printed samples (AlSi10Mg) can be very well protected against corrosion (surface corrosion as well as pitting) by cerium-alloyed active corrosion protection layers made of APPD coating. However, as stated in the literature, sufficient time is required for the very low cerium content (< 1 %) dissolved in the coating to have an active effect against attack by chloride ions from the electrolytes used, i.e. around 24 hours. Short-term tests without a long preceding ageing period therefore only provide representative results to a limited extent, whereas salt spray tests close to the application, as well as ageing tests, correlate very well. As the process can also be used on substrates with a very pronounced 3D topography (i.e. at mm and cm level) using available robot-supported system technology, the APPD application of active wear protection coatings can be easily and economically (high coating rates) transferred to industrial batch coating.
coating can be transferred.
Acknowledgments
Thanks are due to the funding body of the transnational project "APKOLE" especially in Austria, i.e. Österreichische Forschungsförderungs Ges.m.b.H. (FFG), and to the Horizon Europe project MIMOSA (project no. 101091826).
Literature
[1] Abele, E.; Reinhart, G.: Future of Production Munich, Hanser, 2011
[2] Aboulkhair, N.T.; Everitt, N.M.; Ashcroft, I.; Tuck, C.: Additive Manufacturing, 1, 2014, 77-86
[3] Bardon, J.; Bour, J.; Aubriet, H. et al: Galvanized Steel by Atmospheric Pressure Dielectric Barrier Discharge Plasma, Plasma Processes and Polymers, 4, 2007, 445-449
[4] J. Bardon, J. Bour, D. Del Frari, eta l., Plasma Processes and Polymers 6 (2009) 5655-5659
[5] Beier, O.; Pfuch, A.; Horn, K. et al.: SiOx thin films with embedded nanoparticles for surface functionalization, Hybrid Materials 2011, 2nd International Conference on Multifunctional, Hybrid and Nanomaterials, Strasbourg, France, 6-10 March 2011
[6] Beier, O.; Pfuch, A.; Horn, K. et al.: Plasma Processes and Polymers, 10, 2013, 77-87
[7] Brandl, E.; Heckenberger, U.; Holzinger, V. et al.: Materials & Design, 34, 2012, 159-169
[8] Cabrini, M.; Lorenzi, S; Pastore, T. et al.: Corrosion resistance of direct metal laser sintering AlSiMg alloy. Surface and Interface Analysis, 48, 2016, 818-826 and Cabrini, M.; Lorenzi, S.; Pastore, T. et al. Journal of Materials Processing Technology, 231, 2016, 326-335
[9] Caulfield, B.; McHugh, P.E.; Lohfeld, S.: Journal of Materials Processing Technology, 182, 2007, 477-488
[10] Davis, J.R. (Ed.). Corrosion of aluminum and aluminum alloys, Asm International, 1999
[11] Dembele, A.; Rahman, M.; Reid, I. et al.: Journal of nanoscience and nanotechnology, 11, 2011, 8730-8737
[12] O'Dell, J.S. et al, 13.4, 2004, 461-467
[13] Drummer, D.; Drexler, M.; Wudy, K., Procedia Engineering, 102, 2015, 1908-1917
[14] Fanelli, F.; Fracassi, F., Plasma Chemistry and Plasma Processing, 34, 2014, 473-487
[15] Del Frari, D.; Bour, J.; Bardon, J. et al: Journal of Nanoscience and Nanotechnology, 10, 2010, 2611-2619
[16] Del Frari, D.; Bour, J.; Bardon, J. et al.: Organosilicon plasma polymer coatings filled with Ce-based nanoparticles: Characterization of anti-corrosion properties, in: European Federation of Corrosion Publications, No. 58, Self-healing properties of new surface treatments, Edt.: L. Fedrizzi, W. Fürbeth, F. Montemor, Maney Publishing, Suite 1C, Joseph's Well, Hanover Walk, Leeds LS3 1AB, UK
[17] Frank, D.; Fadel, G.: J Intell Manuf, 6(5), 1995, 339-45
[18] Herbert, P.A.F.; O'Neill, L.; Jaroszynska-Wolinska, J. et al, Plasma Processes and Polymers, 8, 2011, 230-238
[19] Heinzl, J.; Harnisch, J.; Irlinger ,F.,;Hoffmann, H.; Petry, R.; Stanchev, S.; Ulrich, C.: Technologies for the production of individualized products. In Individualized Products - Mastering Complexity in Development and Production, Springer Berlin Heidelberg, 2006, 89-113
[20] Herbert, P.A.F.; O'Neill, L.; Jaroszynska-Wolinska, J., Chemistry of Materials, 21, 2009, 4401-4407
[21] Final report: Generative manufacturing of aluminum components for series production, Fraunhofer ILT, 2010
[22] Precisely adapting surfaces, JOT 07/2012, 32-33
[23] Kakaroglou, A.; Nisol, B.; Hauffman, T. et al, Surface and Coatings Technology, 259, 2014, 714-724
[24] Kron, J.; Deichmann, K.-J.; Rose K.: Sol-gel derived hybrid materials as functional coatings for metal surfaces; in: European Federation of Corrosion Publications, No. 58, Self-healing properties of new surface treatments, Edt.: L. Fedrizzi, W. Fürbeth, F. Montemor, Maney Publishing, Leeds LS3 1AB, UK
[25] Leuders, S.; Lieneke, T.; Lammers, S. et al, Journal of Materials Research, 29, 2014, 1911-1919
[26] Leon, A.; Shirizly, A.; Aghion, E., Metals, 6, 2016, 148
[27] Li, W.; Li, S.; Liu, J., Materials Science and Engineering: A, 663, 2016, 116-125
[28] Maskery, I.; Aboulkhair, N.T.; Tuck, C. et al.: Fatigue performance enhancement of selectively laser melted aluminum alloy by heat treatment, In 26th Annual International Solid Freeform Fabrication Symposium, Austin, Texas, USA, 2005, 1017-1025
[29] Michel, M.; Bour, J.; Petersen, J., Fuel Cells, 10, 2010, 932-937
[30] Musa, A.Y.; Mohamad, A.B.; Kadhum, A.A.H. et al, Int. J. Electrochem. Sci, 6, 2011, 5052-5065
[31] Mubarak, N.; Hu, J.; Tang, S.: Electrochemical Study of Unmodified and Inhibitor Doped Silane Films for Corrosion Protection of AA2024-T, In IOP Conference Series: Materials Science and Engineering, IOP Publishing, Vol. 230, No. 1, 2017, September, 012045
[32] Nisol, B., PhD, Chemistry, ULB, Brussels, 2011
[33] Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W., Progress in Materials Science, 74, 2015, 401-477
[34] O'Neill, L.; Herbert, P.A.F.; Stallard, C. et al,. Plasma Processes and Polymers, 7, 2010, 43-50
[35] Osório, W.R.; Goulart, P.R.; Garcia, A., Materials Letters, 62, 2008, 365-369
[36] Pepe, A.; Aparicio, M.; Ceré, S. et al, Journal of Non-Crystalline Solids, 348, 2004, 162-171
[37] Pfuch, A.; Horn, K.; Schmidt, J. et al, Jahrbuch Oberflächentechnik, vol. 66, ed. R. Suchentrunk, Leuze Verlag Bad Saulgau, Germany, 107-113
[38] Pfuch, A.; Beier, O.; Spange, S.; Gerullis, S.; Wiegand, C.; Horn, K.; Volokitin, G.G.; Grünler, B. Schimanskiet, A.: Composite thin films made by atmospheric pressure plasma CVD for bactericidal applications, 12th International Conference Gas Discharge Plasmas and Their Applications (GDP 2015), Tomsk, Russia, September 6-11, 2015, published in Izvestia Vyshich Uchebnych Zavedeniy. Fizika v58 (9/3), 2015, 32-35
[39] Prashanth, K.G.; Debalina, B.; Wang, Z. et al, Journal of Materials Research, 29, 2014, 2044-2054
[40] Revilla, R.I.; Liang, J.; Godet, S. et al, Journal of The Electrochemical Society, 164, 2017), C27-C35
[41] Soukup, L.; Hubicka, Z.; Churpita, A. et al, Surface and Coatings Technology, 169, 2003, 571-574 and Song, G.L.; Liu, M., Corrosion Science, 72, 2013, 73-81
[42] Spange, S.; Pfuch, A.; Wiegand, C., Journal of Materials Science: Materials in Medicine, 26 (2), 2015, 76
[43] Strano, G.; Hao, L.; Everson, R.M. et al, Int J Adv Manuf Technol, 66(9-12), 2013, 1247-54
[44] Tiringer, U.; Durán, A.; Castro, Y., Journal of the electrochemical society, 165, 2018, C213
[45] Uygun, A.; Oksuz, L.; Yavuz, A.G, et al, Current Applied Physics, 11, 2011, 250-254
[46] http://www.wieland.de/internet/de/produkte_und_loesungen/oberflaechen/Beschichtungen.jsp
[47] Xhanari, K.; Finšgar, M., Arabian Journal of Chemistry, 2016
[48] Yang, H.J.; Hwang; P.J.; Lee, S.H. et al, Int J Mach Tools Manuf, 42, 2002, 1203-12
[49] Zheludkevich, M.L.; Raps, D.; Hack, T. et al. Self-healing anticorrosion coatings, in: European Federation of Corrosion Publications, No. 58, Self-healing properties of new surface treatments, Edt.: L. Fedrizzi, W. Fürbeth, F. Montemor, Maney Publishing, Leeds LS3 1AB, UK
[50] Zimmermann, R.; Pfuch, A.; Horn K. et al, Plasma Processes and Polymers, 8, 2011, 295-304


