Part 2 of our monthly cleaning series looked at the layers of dirt, identified different types of dirt and cleaning agents and defined the task of surface cleaning. This part now goes into more depth, identifies weaknesses in surface cleaning, defines cleanliness and looks at the separation of the bonds between the surface and particles.
Obstacles and barriers to previous cleaning technologies for wet chemical surface cleaning
On the surface of a component, temporally and spatially defined purity is maintained. This is always an active process controlled by humans and their machines, which can be maintained to a limited extent in terms of time and space. The cleaning technologies currently used in series production are processes based on wet chemistry. Water-soluble cleaners with pH values ranging from "acidic" to "strongly alkaline" are used. The most common applications work in the pH value range of 7 to 9. The chemical additives in the cleaner are selected depending on the material. At the end of the cleaning process, a passivation film remains, which seals the component surface cleaned of foreign substances against undefined contamination. Corrosion or reduction processes are prevented or minimized. In every case of cleaning, chemically harmful residues are formed which require special treatment during disposal. Very large quantities of water are used as a solvent in these processes and therefore require enormous procurement efforts and costs. Component cleaning is cost-intensive and requires comparable energy expenditure per component as in machining. Each type of component requires its own cleaning technology. The time and costs involved in previous cleaning technologies are increasing, as the requirements for the results of cleaning and the amount and size of foreign matter remaining on the component surface must be reduced. The component properties in the areas of automation and in the areas of modern machines and systems demand this.
The component-bound technologies of cleaning chemistry hinder the multiple use of cleaning systems. The mostly open energy cycle causes expanding costs. Closed energy cycles with low energy emission to the environment are the exception so far. The automatic monitoring of chemical concentrations in the cleaning solution, consisting of water, cleaning agent, cooling lubricants, chips, dirt particles of all kinds, etc., has so far been inadequately solved. Only manual, personal laboratory analysis provides evaluable results. Automated measuring techniques are comparative measurements that can be evaluated more or less depending on the operator. Comparative control systems are mostly used.
|
Obstacles and barriers |
Workarounds and detours of previous cleaning technologies |
|
Component material - dependent addition of chemical cleaning concentrates |
Dosing with concentration monitoring, usually conductivity measurements or proportional addition in relation to fresh water consumption, particle counters, ink tests, water breakage test, pH value monitoring, water hardness testing, regular titrations in the laboratory, manual re-dosing or correction of concentrations, complex preparation of the media |
|
Type of contamination is treated with a type of broadband solution |
Different chemical additives from acidic to alkaline, mechanical pre-cleaning such as ultrasound or brushing, duration of cleaning, extended exposure times, high-pressure water jets |
|
Media dosing, media consumption |
Generate water consumption through evaporation and carryover in order to achieve the inflow of fresh water to dilute the cleaning solution, separation of dissolved foreign particles through filtration, bath heating and thus component heating in order to utilize the higher solubility of warm water and enable drying of the components, high energy consumption as mostly open energy chains, detergent re-dosing |
|
Component-specific cleaning technology and special cleaning system |
The creation of a modular system of cleaning system technology, the change of "tools" and media depending on the component |
|
Control susceptible, with large tolerance, operator monitored |
The creation of a modular system of cleaning system technology, the change of "tools" and media depending on the component, the control susceptible, with large tolerance, operator supervised by specialist personnel, the control according to the test plan of the parameters, the limited multi-machine operation, the generously specified limit values, the small number of samples in laboratory quality, the preventive water change and the oversizing of the system aggregates such as pumps, heating and blowers. |
The obstacles in practice, such as strongly fluctuating chemical-physical parameters of the detergent solution, inadequate measurement technology, lack of or too few qualified personnel, random sample control of the cleaning results with run times of 4 to 10 hours per sample, special evaluation technology and evaluation rooms in laboratory quality, allow only conditionally reproducible results of the cleaning processes, see Table 1. The hourly control of the cleaning solutions, washing and rinsing solutions, which is to be aimed for, is hardly ever carried out due to the time and material expenditure. The tolerance range of the cleaning results of the process requires more special efforts. The large number of parameters to be controlled makes it difficult to maintain the consistent quality of the production process.
The definition of purity
Up to now, direct measurement has only been possible as a defined random sample. Defined partial areas on the component surface are viewed through a microscope and the foreign particles are measured and counted. Alternatively, component surfaces are cleaned in a defined manner using a cleaning solution, the draining medium is poured over a test filter and the result is then evaluated using the specified limit values. Absolute cleanliness is a theoretical value. The measuring equipment used to determine the foreign particles determines the value. A testable, reproducible value is determined from the design and technology of the component.
Statements on the foreign particles are specified. The environment of the components has a direct effect on the condition of the contamination. If the cleanliness of the surface is not to exceed a value of foreign particles, the environment of the components must be 10 times "cleaner", i.e. all parameters may only have a maximum of 1/10 of the values of the foreign particles. In practice, particles smaller than 100 µm in length are counted in order to also record the possibility of clumping. The particles are classified.
The cleaned component surface is exposed to oxygen, i.e. the surface will oxidize. This process can be prevented or delayed by applying a passivation film. This passivation film is always on the component surface as a result of the cleaning process. The "clean" component is defined as unclean.
So when talking about cleanliness, the question of definition must always be answered in a measurable, reproducible way with a quantified statement.
The aim is to remove foreign particles of metallic material from the component surface. The size of the particles is between 50 µm < length < 1000 µm of solid particles, between 10 µm < width < 1000 µm of solid particles with a material thickness of greater than 10 µm. Particles with a larger size are usually clamping chips that cannot be removed or cannot be removed reproducibly.
Fibers up to 5 mm in length are removed from the component surface. Clusters of fibers above a certain size and mass are removed irregularly.
The rules according to VDA 19 and the rules according to ISO 16 232 /01...10 are used as the basis for testing and evaluating the cleanliness of the component surfaces.
The separation of physical bonds
It is not "hooks and eyes" that make up a connection. It is not mechanical, form-fit bonds that connect foreign particles to a component surface. It is the properties of the atoms and electrons, the free ions and their interactions with each other. Attractive forces are formed here depending on the parameters of the particles.
The separation of foreign particles, the elimination of adhesion, means the discharge of the compound, the elimination of the attractive forces with subsequent mechanically induced transport of foreign particles.
Electrical surface discharge can take place in three ways. The base material and the type of earthing have a significant influence on the speed of the surface discharge. Metallic materials generally have good electrical conductivity and low contact resistance to earth. The layering of the processed layers after processing forms a contact resistance to the base material, indeed a form of insulation of the external charge to the conductive base material. The ambient radiation and the surrounding media, gaseous or liquid, contribute to the surface discharge of the body with their electrical charge.
Quantification of the binding energy between the object surface and foreign particles
The OH groups can be present as O and OH groups, i.e. negatively charged or neutral. As a chemical equilibrium will occur, the concentration of O- (i.e. the surface charge) is a function of the concentration of H+ in the solution (i.e. the pH value). The higher the H+ concentration, the lower the pH value, the fewer O- groups are present.
Model concept
Oppositely charged ions attract each other, identically charged ions repel each other. For these reasons, ions are not randomly distributed in a solution, but have a certain close order in which anions tend to be found near cations and vice versa. Electroneutrality of the solution is maintained. In contrast to the ion lattice, ions cannot arrange themselves completely regularly in solution because solvent molecules as dielectrics weaken the Coulomb interactions, whereupon the thermal movement leads to a stronger distribution of the ions. On average over time, however, each ion is located in the center of a cloud of oppositely charged ions (indicated by circles in Figure 1 ). These ion clouds shield the charge of the central ion, which is the reason for the introduction of activity as an "effective concentration" for ions [1].
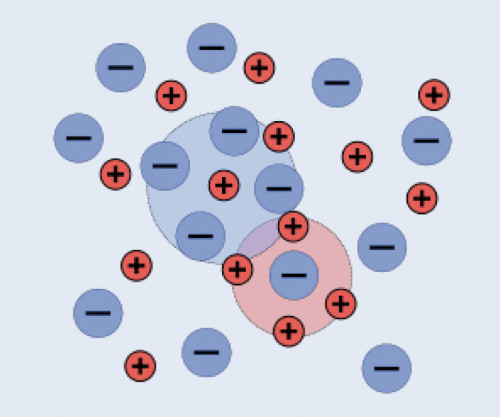 Figure 1: Ion distribution in a solution
Figure 1: Ion distribution in a solution
"Based on these model concepts, P. Debye and E. Hückel derived some equations frequently used in electrochemistry by combining the Poisson equation with Boltzmann statistics to describe the ion distribution. To simplify matters, they used the following quantities
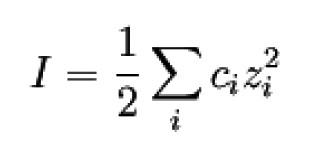
Ion strength I
ci Concentrations of the ionic species in the solution
zi Charge numbers of the ionic species
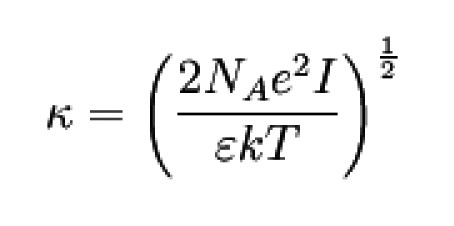
e Elementary charge
ε Permittivity of the solvent;
k Boltzmann constant (see Physical constants)
T Temperature
NA Avogadro constant
Activity coefficient f of the ionic species i
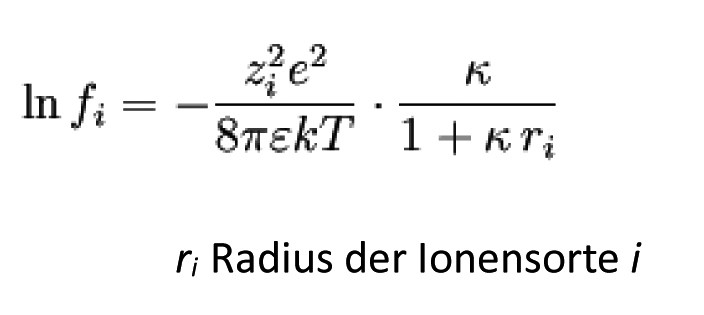
This equation is often used as an extended
Debye-Hückel limit law in the form
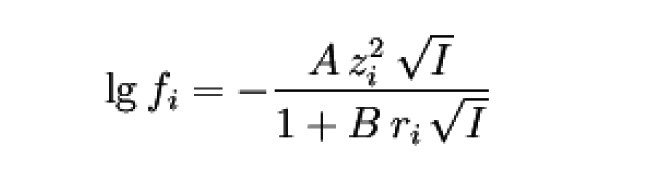
form. In it are
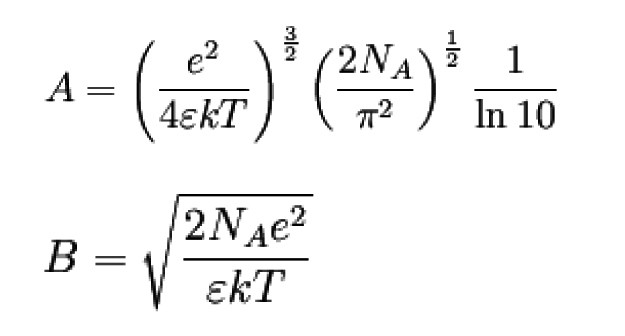
The range of validity is approximately mol dm-3.
Radius of the ion cloud
It turned out that the inverse of κ can be interpreted as the radius of the ion cloud.
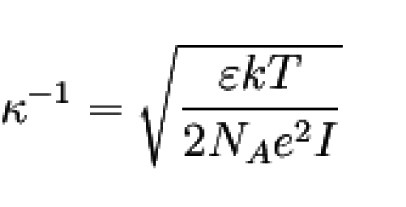
This radius is also called the shielding length or
Debye length.
Debye-Hückel limit law
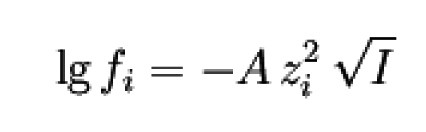
A = 0.509 dm3/2 mol-1/2 if water at 25 °C is used as the solvent. For other temperatures and/or solvents, it must be calculated according to the equation given above.
This is the most frequently quoted equation for practical purposes, which results in the approximation. It therefore applies to ion clouds that are considerably larger than the enclosed ion. As a rule, these are very dilute solutions with mol dm-3.
Mean activity coefficient and Debye-Hückel limit law
Although individual activity coefficients (or activities) can be calculated, they cannot be measured due to the electroneutrality condition. The following applies to the measurable mean activity coefficient of an electrolyte
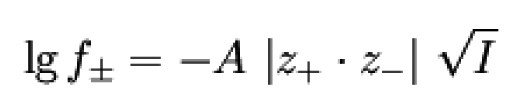
Literature on this in [2][3].
Debye-Hückel-Onsager theory
A refinement of the Debye and Hückel model is the Debye-Hückel-Onsager model. In this model, hydrodynamics are also taken into account. Unfortunately, this makes the model mathematically more complicated. A detailed derivation would go too far within the scope of this learning unit, which is why the model will only be presented briefly.
When an external field is applied, the central ion and the ion cloud move in opposite directions. The ion cloud must always be reconstructed. This results in a relaxation effect, i.e. additional friction is caused. Furthermore, the centers of charge of the central ion and the ion cloud are shifted (electrophoretic effect). This additional braking effect must be added to Stokes' friction. The final equation is as follows:
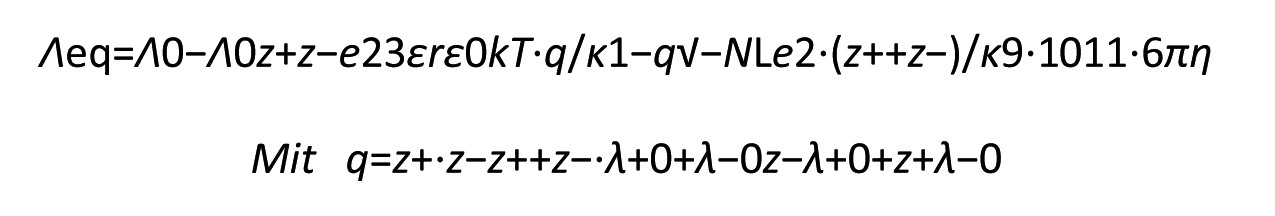
For strong (1:1) electrolytes, the equation simplifies to:
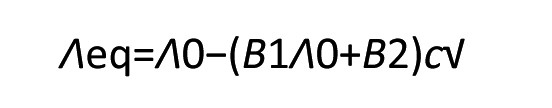
This equation agrees with the empirically found Kohlrausch's square root law. A detailed analysis of the microscopic relationships to conductivity is particularly necessary for frequency-dependent investigations (impedance measurement) [4].
In contrast to electrostatic forces in everyday life, which can act over large distances (e.g. lightning), the range of electrostatic forces in the systems investigated is very small. The reason for this is the shielding of charges by counterions from the surrounding solution, described by the Debye-Hückl theory.
Measuring surface charges is not particularly easy and requires a few tricks, especially in ionic solutions. Physical methods can be used in air, such as the force on a vibrating charged plate near the surface to be examined (force in the plate capacitor). If the surface is electrically conductive, a voltmeter could be connected directly, but many surfaces of interest to mechanical engineers are electrical insulators. Other techniques are therefore required. Some of the few universally applicable methods are current potential measurements and methods that directly measure forces, such as the force microscope or the SFA (surface force apparatus). In this work, no force measurements were carried out using a force microscope. Various measurement series from the literature were read and the results I evaluated were used for the objectives of this thesis.
Polarity and water solubility - emulsions and their structures in cooling lubricants
Emulsions are defined as at least two immiscible liquids. One liquid predominates in quantity and the others are usually finely dispersed in the first-mentioned liquid, not dissolved. The liquids are mechanically moved towards each other, stirred. If the movement stops, the components of the emulsion separate. The addition of an emulsifier can delay segregation, but complete segregation cannot be prevented in liquids at rest.
In the case of water in oil emulsions, the oil phase is continuous and the water phase is distributed in small droplets (example: spreadable fats). The water content of water in oil emulsions must not exceed 74%. In the case of oil-in-water emulsions, the proportions are reversed: the water phase is continuous and the oil phase is distributed in small droplets (example: cooling lubricants). An oil in water emulsion must contain at least 26 % water. If there is more than 6 % oil by volume dissolved in water, an oil layer begins to form on the surface of the liquid. The emulsifier as an aid to the stable distribution of various liquids are surfactants. Surfactants can be composed of a hydrophilic (water-friendly) and/or a hydrophobic (lipophilic, fat-friendly) part.
The hydrophilic part attaches itself to the water at the interface between oil and water, while the hydrophobic part is in the oil (fat). A distinction is made between oil-in-water (O/W) and water-in-oil (W/O) emulsifiers. The emulsion type (O/W or W/O) is not determined by the water content of the emulsion, but by the emulsifier used. Emulsifiers in which the water-friendly groups predominate lead to oil-in-water emulsions. If, on the other hand, the fat-friendly groups predominate, water-in-oil emulsions are formed [5].
Substances that consist of ions or molecules that contain charges are called polar. Molecules without these charges are called non-polar. The water molecule is polar because its two hydrogen molecules have a positive partial charge and its oxygen molecule has a negative partial charge. Since like dissolves in like, polar substances (e.g. salts) are more soluble in water than non-polar substances such as oil [6]. Cooling lubricants from machining processes are removed from the component surface using the method found in this paper.
Literature
[1] https://de.wikipedia.org/wiki/Debye-H%C3%BCckel-Theorie
[2] Berson, J. A.: Chemical Creativity - Ideas from the Work of Woodward, Hückel, Meerwein, and Others, Wiley-VCH, Weinheim 1999
[3] www.chemie.de/lexikon/Debye-H%C3%BCckel-Theorie.html
[4] www.chemgapedia.de/vsengine/vlu/vsc/de/ch/13/vlu/echemie/leitfaehigkeit/leitfaehigkeit.vlu/Page/vsc/de/ch/13/pc/echemie/leitfaehigkeit/onsager.vscml.html
[5] www.chemie-master.de
[6] www.lenntech.de/element-und-wasser/loeslichkeit.htm#ixzz2riD7KbzY)


