Miniaturized components and metal components with free geometric shapes (e.g. bionic components) pose great challenges for electrochemical processing. In addition to industrial dispersion deposition and layer composites as well as the deposition of more complex alloy systems, processes with dynamic current control during the electrochemical process, such as pulse anodizing, plasma electrolytic oxidation, electrochemical processing and shearing, are suitable here.
Changing industrial requirements are always a strong driving force for optimization and development processes. This also applies to the more traditional field of electrochemical and galvanic surface treatments. In the past, the focus was primarily on improved properties in terms of hardness, wear resistance and corrosion resistance.
Electroplated coatings have been further developed in this direction, characterized in particular by a transition from pure metal deposition to alloy deposition. These systems are now industrially established and mostly standardized. An initial rethink occurred as a result of the rapid increase in regulatory changes motivated by environmental policy, which restricted the use of proven systems and, in addition to a relocation of production to non-European third countries, also led to a rethink in the use of certain metals and bath ingredients.
New applications and product generations require additional surface properties. These include improved chemical stability, biocompatibility, a superficial microstructure, sliding properties (the subject of self-lubricating surfaces), magnetic properties and lateral heat or shock dissipation. Electroplated coatings as well as anodic passivation must be adapted to these requirements. In addition, miniaturized components pose further challenges for electrochemical processing. Such requirements and their large-scale industrial realization already require a certain rethinking in electroplating production, but recently producible metal components with free geometric shapes (e.g. bionic components) require new surface treatment concepts.
In addition to the industrial use of dispersion deposition and layer composites as well as the deposition of more complex alloy systems, the key to enabling such additional tasks is above all the use of dynamic current control during the electrochemical process. Classic thermodynamically controlled processes (direct current deposition, classic anodizing or electropolishing) are increasingly reaching their limits and must be replaced by kinetically controlled, dynamic processes. What all these processes have in common is an increased requirement for current and potential control, which means that a deeper understanding of the electrochemical processes taking place on the surface of the component is an absolute prerequisite. This is the only way to adjust pulsed processes precisely to the component and the surface requirements.
The most prominent representative of dynamic electrochemical processes is certainly pulse deposition. However, there are several other interesting processes that will be presented in this chapter. These include pulse anodizing, pulsed plasma electrolytic oxidation (PEO), electrochemical machining (ECM) and the recently established process of sheathing.
Pulse plating
Pulse plating is the use of a modulated current for electrolytic metal deposition. Most frequently, rectangular pulses interrupted by so-called pulse pauses are generated using suitable pulse rectifiers. These cathodic pulses (metal deposition) are supplemented by one or more anodic current pulses switched at regular intervals (metal dissolution). A constantly repeating sequence of cathodic and anodic pulses with the corresponding pulse pauses (time-out) represents the so-called pulse sequence [1]. The rapid development in the field of computer and electronic systems has led to new possibilities for visualizing potential fields and field line distribution as a function of the applied current; the new possibilities in high-performance electronics now allow the error-free implementation of fast pulses via suitable rectifier control. This enabled the development of pulse plating processes based on effects in the regime of secondary current density distribution (under kinetic control) with a much better application possibility in industrial production.
Several material properties of the deposited metal can be specifically changed by pulse plating. This is always based on a variation of the electrochemical conditions by adjusting the individual pulse parameters during deposition. With direct current deposition, the process can only be varied via the amount of current used for deposition (via the average current density). In contrast, with pulse or reverse pulse deposition, the user has a considerable set of different parameters available for process optimization. In addition to the average current density, these are the cathodic and anodic pulse current density, the cathodic and anodic pulse duration, the length of the pulse pause and the pulse frequency. Derived variables such as the load cycle (ratio of the sum of the pulse durations per sequence to the sum of the length of the pulse pauses per sequence) and the ratio of anodic to cathodic current per sequence are further important influencing variables on the pulse deposition process. Many of these parameters cannot be varied completely independently of the others, as they partly influence each other. This list does not yet take into account the possibilities of complex pulse sequences. For example, the average current density in reverse pulse deposition alone depends on the average current density jav, the cathodic pulse current density jkath, the anodic pulse current density janod, the cathodic pulse duration tkath, the anodic pulse duration tanod and the duration of the pulse pausetoff.
This abundance of relevant parameters makes a purely empirical solution approach almost impossible. However, precise knowledge of the electrochemical principles allows a knowledge-based and therefore much more efficient solution to this multidimensional matrix. In particular, the electrochemical kinetics during pulse current deposition, the time required for charging and discharging the electrolytic double layer at the electrode (the workpiece to be coated), mass transport limitations (pulse limiting current density), current density distribution and crystallization effects must be taken into account. Numerical simulation programs for calculating the electrochemical conditions in the deposition tank (e.g. potential field and current density distribution) are very helpful in determining the parameters and thus in optimizing the pulse processes. Understanding the electrochemical principles of the electrolyte system used is an essential step in this process. For this reason, special importance was always attached to this aspect in all work. Investigations on a rotating electrode represent the core of the electrochemical work.
The maximum pulse frequency influences the structure and properties of the deposited metal layer. Due to the increasing relevance of nanotechnology in particular, various research groups have been striving for the finest possible metal deposit with primary crystallite sizes in the nanometer range. The often published extremely short pulse times actually produced "nanocrystalline" metal layers on a laboratory scale under precise adherence to complex experimental conditions. In practice, however, the time required to charge (and discharge) the electrolytic double layer determines the maximum pulse frequency (and therefore the minimum possible pulse duration). If the time falls below this limit, the applied rectangular pulses on the electrode surface (the workpiece to be coated) will be significantly distorted. This would make pulse deposition uncontrollable and difficult to reproduce. For this reason, the process and the pulse sequence must be defined in such a way that the time in which the double layer is charged and discharged is much shorter than the pulse duration or the pause after the pulse. The charging and discharging times must be determined separately for each electrolyte system.
The limitation due to mass transport is based on the depletion of the cations in the diffusion layer on the electrode (workpiece) surface. Pulse deposition can directly influence the structure and thickness of the diffusion layers. A distinction can be made between two different diffusion layers during pulse current deposition. In the immediate vicinity of the cathode, the metal ion concentration fluctuates to the rhythm of the pulse frequency; this is referred to as a pulsating diffusion layer. This is followed by another diffusion layer with a constant concentration gradient (stationary diffusion layer) towards the inside of the solution. The depletion of cations in the pulsating diffusion layer limits the pulse current density, while the depletion in the outer diffusion layer limits the average current density. The practical current density can therefore be slightly increased in comparison to direct current; a further increase in the average current densities is only possible by manipulating the current density distribution during reverse pulse deposition.
Pulsed current deposition also allows the targeted modification of the deposit properties, which depend on the deposited microstructure. The deposit structure is determined on the one hand by the formation of the primary crystallites of the deposited metal and on the other hand by the further growth of these crystallites. The ratio of crystallite formation and crystal growth can be influenced by the pulse deposition (e.g. via the pulse current density, the pulse frequency or the load cycle). The general layer structure (columnar/fine crystalline/lamellar) can also be changed during pulse deposition. The anodic component in the pulse sequence also plays a significant role here. When the applied current is changed by pulses, the type of adsorbed species can change and, depending on the adsorption rate constant, the surface diffusion changes. This leads to different crystallization mechanisms and properties of the deposited precipitate.
The kinetics of simultaneous reactions have a great influence on the relative speed of competing reactions, e.g. in alloy deposition or hydrogen evolution [1]. By influencing the deposition kinetics via the shape and sequence of the applied current pulses, alloy compositions and phases can be manipulated and side reactions suppressed. The pulse pause also plays an important role in the reduction of the resulting hydrogen. During the current pauses, adsorbed substances or gas bubbles can desorb, especially if there is good bath movement at the same time.
The classic and probably best described application of pulse deposition is printed circuit board production. The copper system is used as a model substance. Due to the enormous growth figures in the last two decades (Asia!), the chemical industry has also made a not inconsiderable contribution to optimizing this system by optimizing the electrolytes. Fully automated production lines with exact bath guidance and precisely defined (horizontal) electroplating cells long represented the pinnacle of pulse deposition.
In the slipstream of this development, there were repeated attempts to use pulse deposition in other areas as well. For the last 20 years or so, the possibilities of pulse deposition have been used for precious metal deposition in particular. The driving force here has always been material savings with mostly improved coating properties. For example, the use of reverse pulse deposition makes it possible to significantly reduce the minimum layer thickness of contact and protective layers made of gold. This is very successful not only in the rack but especially in the strip process.
One application of pulse deposition that often goes unnoticed by the general public is the production of hard disks. Precisely defined pulse deposition processes are used to produce magnetic multilayer coatings, which then act as the actual carriers of the stored information.
Due to the rapidly changing raw material prices, particularly for nickel, pulse deposition is also becoming increasingly popular in other areas. For example, there are industrial applications in individual copper deposition processes and in functional nickel plating to increase layer uniformity and thus save raw materials. Increasing production capacity while maintaining the same coating properties is also a possible application and is a major driving force behind the switch to pulse deposition, even with the currently significantly lower raw material prices.
Tribologically active coatings can be achieved by pulse deposition of alloys. This effect can be further enhanced by incorporating micro- or nanoparticles. In addition to classic applications such as the incorporation of macroparticles in grinding tools and micro- to nanoparticles in wear-resistant surface coatings, active particles are increasingly coming to the fore. This can be the production of a catalytically active surface or the incorporation of active substances in the field of antiseptic/antibacterial surfaces. All investigations carried out in this work on the combination of electrochemical pulse deposition with the incorporation of different particle classes are based on the elucidation and adjustment of the zeta potential of the inert particles in the electrolyte.
The application of pulse deposition also appears to be particularly interesting in the field of strip electroplating, where, for example, increased uniformity (improved layer thickness distribution) of the deposited layers immediately leads to a significant savings potential for the precious metals often used in this area (micro-contacts and similar). The advantage of numerical process simulation can also be used particularly well here thanks to the precisely fixable cell geometries.
Hard chrome layers are probably the most prominent representative of such tribologically relevant galvanic surfaces. Crack-free hard chrome layers can be produced using pulse processes, as can microstructured surfaces. Especially with regard to replacement coatings for hard chrome plating, all common chrome-free alternatives are alloy coatings. Some of these alloys cannot be produced using direct current deposition, or cannot be produced in a meaningful way. By appropriately controlling the alloy composition and increasing the hardness through targeted structural changes in the layer by selecting suitable pulse conditions, high-performance systems that come close to hard chrome plating can now be used industrially.
The structural influence also has an effect on the ductility of the coatings, which is a decisive coating property, particularly in the field of microplating and microcontacts. Very dense structures are ideal as barrier layers.
In general, pulse deposition is most successful where the focus is on the special functionality of the electroplated layers. Magnetic layers are not only essential for storage media, but also open up a very wide range of applications thanks to the possibility of magnetizing the surface of non-magnetic workpieces. Both soft and hard magnetic systems can be produced and provide the basis for a wide range of sensor and control applications. Alloy systems that are difficult to obtain using direct current can also be produced using pulse deposition.
In general, it is to be expected that further applications will be realized in the short and medium term using pulse deposition. A replacement of DC coatings by pulsed metal coatings is likely in many areas in the long term.
Pulse electropolishing
Electropolishing is an electrochemical method for finishing metals in which surface roughness is reduced and surfaces are smoothed by means of anodic dissolution. Various metals and alloys can be post-processed in this way, and other materials such as silicon can also be successfully electropolished. Electropolishing is usually carried out in still electrolytes based on phosphoric, sulphuric, perchloric or acetic acid. The solvent used in such electrolytes is usually water (in low concentrations) or alcohols such as methanol. Recent developments also show a high performance of molten salts or ionic liquids as media for electropolishing [1].
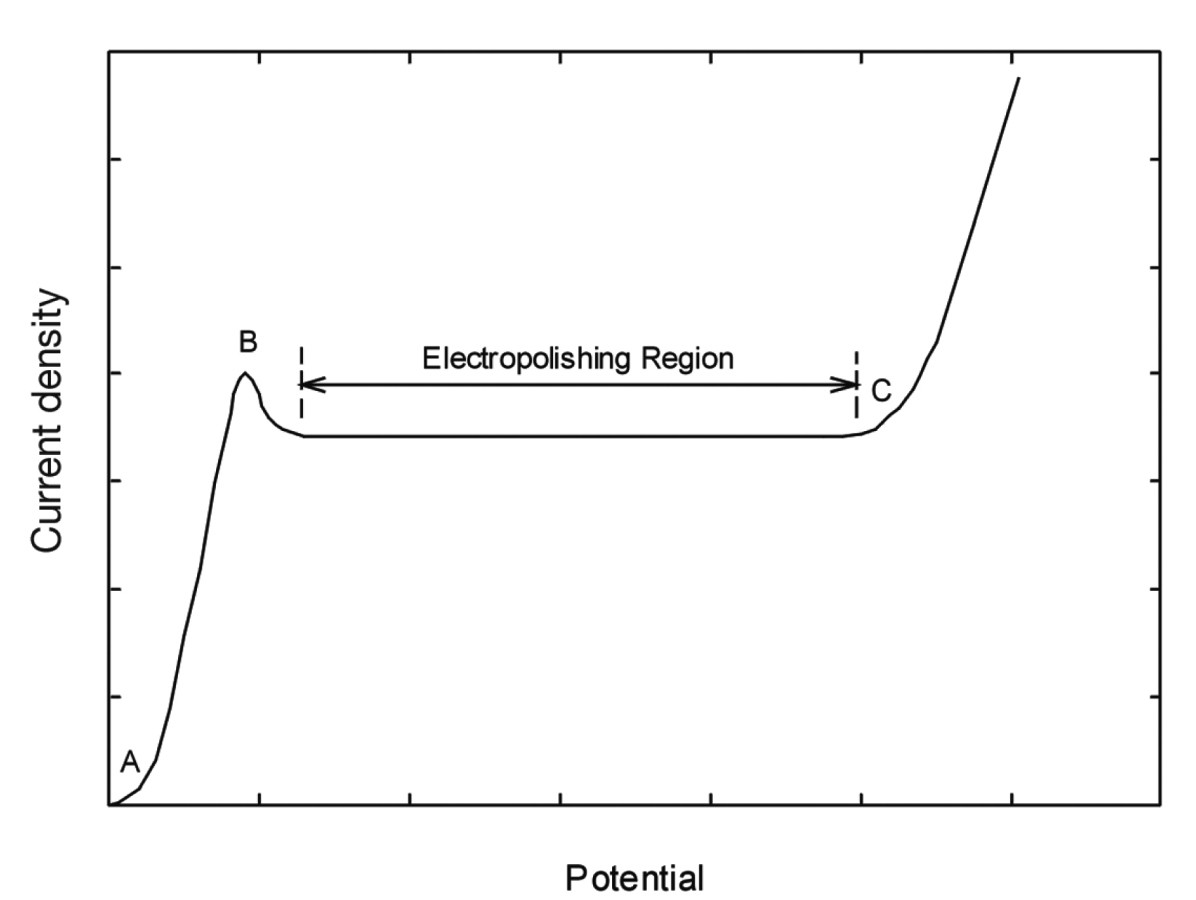 Fig. 1: Schematic representation of the anodic polarization curve for a typical electropolishing electrolyte. The curve section A-B corresponds to the region of active dissolution, the region B-C is the region of anodic film formation. In this region there are limiting current conditions, recognizable by the current plateau that forms
Fig. 1: Schematic representation of the anodic polarization curve for a typical electropolishing electrolyte. The curve section A-B corresponds to the region of active dissolution, the region B-C is the region of anodic film formation. In this region there are limiting current conditions, recognizable by the current plateau that forms
The typical schematic representation of an anodic polarization curve for an electrochemical system is shown in Figure 1. The curve section A-B corresponds to the region of active dissolution, the region B-C is the region of anodic film formation. In this region there are boundary current conditions, recognizable by the current plateau that forms. Within the active region, dissolution occurs preferentially on certain crystal surfaces and defects, which can lead to matt and sometimes rough surfaces. The current plateau that forms is associated with the formation of a surface film. The central effect of such films is the minimization of crystallographic etching influences. This region is the most effective area for electropolishing. Tertiary current density distribution prevails in this region and the process is under diffusion control.
The exact structure of this passive film has not yet been fully clarified; there are several models of differently viscous or compact surface films [1]. The latest theories postulate the existence of a salt film because mass transport control is present in the area of the current plateau [2]. However, a polishing effect is also reported under transpassive conditions [3].
In electropolishing, a basic distinction is made between surface leveling and polishing. The former refers to the removal of coarser roughness (> 1 μm) while the latter aims to minimize unevenness of a much smaller size (< 1 μm). To produce reflective surfaces, the elimination of surface irregularities with a comparable wavelength to visible light (λ < 1 μm) is required. Most industrially relevant applications of electropolishing processes require the combination of these two functions, whereby the mechanisms of both processes (leveling - polishing) are fundamentally different. For polishing, only the formation of the surface film for homogeneous anodic dissolution is required, whereby the prevailing current density distribution is mainly responsible for suitable leveling [1].
The use of pulsed electrochemical methods is based on the same principles as electropolishing. For the same average current density, the current density distribution tends to be more primary in pulse electropolishing. This means that the geometry-dependent leveling effect is stronger than with classic DC-controlled polishing. Under purely tertiary current density conditions, the leveling effect depends primarily on the thickness of the diffusion layer (δ and δp). This layer thickness can be specifically varied by selecting the appropriate pulse parameters. This makes it possible to convert a so-called microprofile into a macroprofile. This is advantageous for pulse deposition, but results in virtually no macro leveling during pulse electropolishing. Under these conditions, however, a high gloss can be produced on the surfaces. In pulse electropolishing, a condition that is rather disadvantageous for deposition must therefore be deliberately created. The targeted adjustment of mass transport control is easily possible using pulsed currents (or potentials). The off-time can also play a certain role, as reaction products are transported away from the surface during the off-time. However, excessively long off-times lead to the dissolution of the anodic polishing film generated during the current pulses. In this case, chemical etching and the associated roughening of the surface would occur [4].
Despite these different options for precisely adjusting the electrochemical polishing conditions, pulse electropolishing is hardly used industrially, or only in niche applications [5, 6]. This may be due to the challenge of precisely setting the individual parameters, which requires a knowledge-based approach. In an industrial environment, the simpler direct current process is therefore usually preferred. The most important areas of application for pulse electropolishing are various forms of stainless steel, primarily for medical technology applications [7, 8]. Another application is mechanically assisted polishing (electrochemical wipe polishing). Here, the state of the tertiary current density distribution between the cyclical processing steps must be adjusted via adapted pulse control. The polishing film is briefly destroyed by the mechanical processing and is then rebuilt by current pulses. This combined process results in high-gloss, smooth surfaces.
In medical technology, pulse electropolishing is used to smooth the surfaces of miniaturized implants such as stainless steel stents. Compared to direct current processes, significantly lower surface roughness can be achieved from sulphuric and phosphoric acid electrolytes [7, 8]. Interestingly, relatively low pulse frequencies (50Hz) are more effective than high-frequency pulses (>1kHz). At high pulse frequencies, the pulse duration is not sufficient to form a homogeneous surface film. A further advantage, particularly on fine microstructures, is that gas bubbles that form are blown off by the current pulses.
Precious metals such as niobium are also electropolished using pulsed processes, particularly for the production of superconducting surfaces for radio frequency conduction (SRF) in high-energy particle accelerators [9].
Since electropolishing is not only used industrially to improve the decorative appearance, but increasingly to improve corrosion resistance and abrasion resistance, the number of applications is generally increasing. With better knowledge of the electrochemical principles and driven by ever increasing demands on component surfaces, it is therefore to be expected that pulse electropolishing will gain in importance alongside classic DC polishing.
Pulse anodizing
Many metals form a passive surface film under anodic potential conditions. This is usually thin (a few nanometers) and counteracts further metal dissolution. Some metals such as titanium, aluminum, zirconium and tantalum behave somewhat differently (see Fig. 2). With these metals, not only does a (thin) natural oxide layer form under atmospheric oxygen, but the thickness of the anodic film can also be further increased to the micrometer scale by applying current or an anodic potential [10]. This process is called anodization. The resulting layers have a low electrical conductivity and also shield transpassive reactions up to higher potentials. Such thick oxide films act as corrosion and wear protection. Colorful, decorative coatings can be produced by adding dyes.
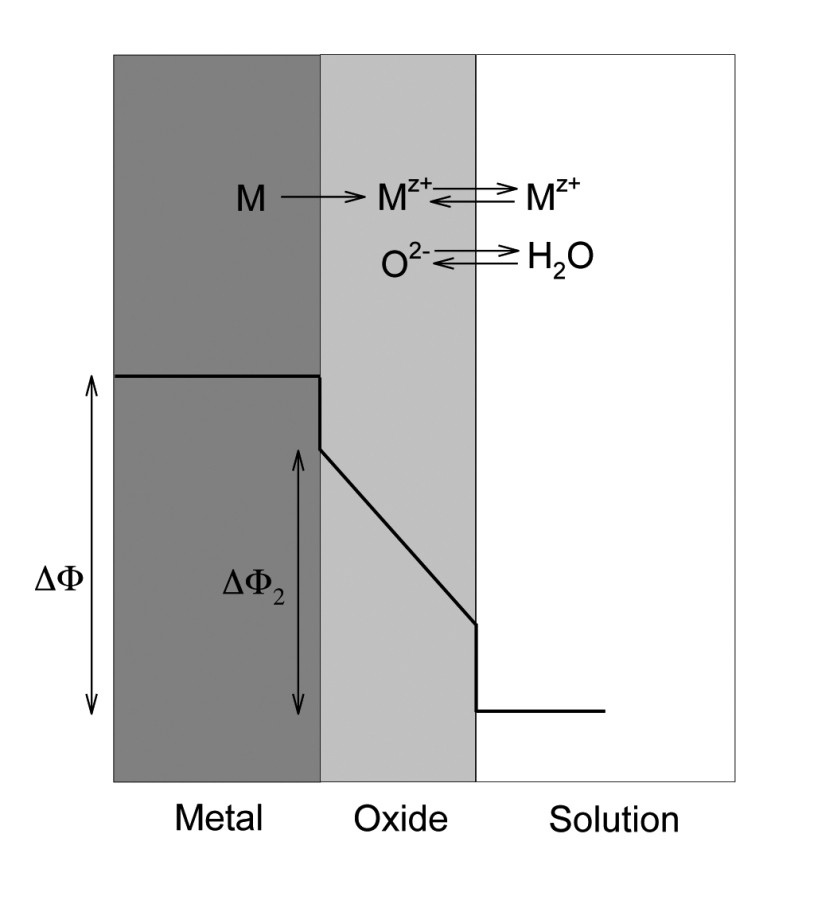 Fig. 2: Schematic representation of the processes occurring during the anodization of a metal surface of the metal M with the corresponding variation of the potential over the cross-section
Fig. 2: Schematic representation of the processes occurring during the anodization of a metal surface of the metal M with the corresponding variation of the potential over the cross-section
The most important industrially used anodizations concern the metals aluminium, titanium and tantalum as well as their alloys. Aluminium and tantalum are used in their anodized form for capacitors, aluminium for various everyday items (from drinking bottles to house facades) and titanium mostly for medical technology products and implants [11-13].
The application of pulsed currents (potentials) during anodization intervenes directly in the build-up mechanism of the layers. Layer growth involves a series of ion transfer reactions at the interface between the metal and the oxide layer and the oxide layer and the electrolyte [14]. Figure 2 schematically shows these reactions in correlation with the potential curve.
The best known mechanistic model is the so-called "high field conduction" model which correlates the growth rate of the oxide film with the movement of the cations in the applied electric field. Under purely potentiostatic conditions (i.e. constant potential), the potential field is reduced with increasing oxide film thickness and the reaction slows down with an exponentially decreasing current flow. From a certain point in time, the current flow is reduced to a negligible level and there is de facto no more film growth. Under constant current (galvanostatic conditions), both the layer thickness and the potential increase linearly as the anodizing process progresses.
The most important anodizing process concerns aluminium and its alloys and is carried out under alternating, direct and pulsed current conditions. In neutral solutions, barrier layers can be produced in this way, which are usually used in the manufacture of capacitors. In acidic and basic media, thicker oxide layers can be produced (Fig. 3). At the beginning of the anodizing process, a thin, very compact layer with a thickness of 20-100 nm is formed. As anodization progresses and the layer grows, the oxide film becomes increasingly porous. This porous film can reach a thickness of up to several dozen micrometers. The resulting layer consists of a system of essentially parallel pores that extend to the initial thin barrier layer. Each pore and the surrounding material form a hexagonal cell, which are arranged into a tight, honeycomb-like lattice structure. The pore diameters are in the range of 10-30 nm [15] and are directly related to the applied potential.
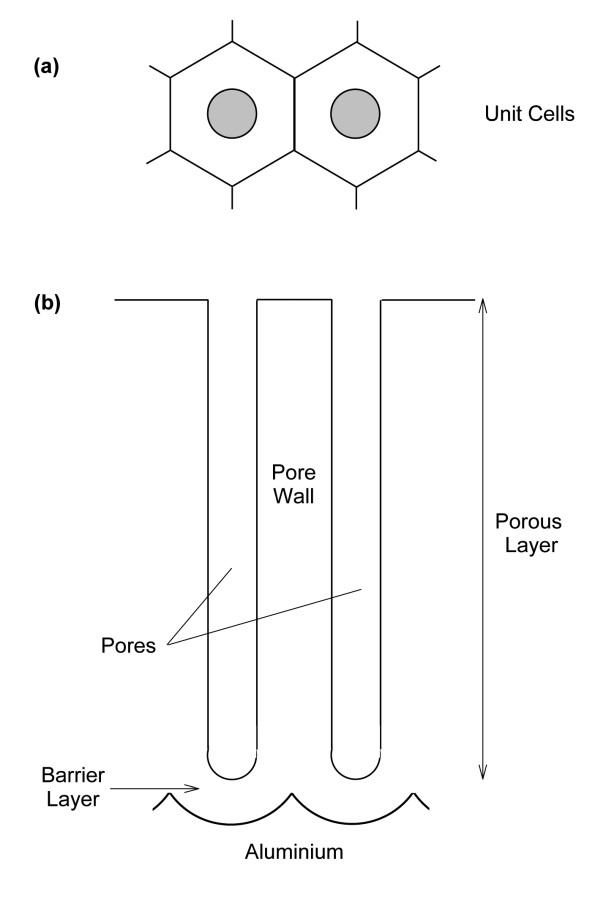 Fig. 3: Idealized structure of a porous oxide film during anodization: (a) bird's eye view with the hexagonal honeycomb structure, (b) cross-section
Fig. 3: Idealized structure of a porous oxide film during anodization: (a) bird's eye view with the hexagonal honeycomb structure, (b) cross-section
After anodizing, the layers can be coloured in different ways, for example by the incorporation of coloured particles or the precipitation of metal salts into the porous structure. The layers are then sealed by converting the aluminum oxide into boehmite by boiling in hot water. The increase in volume resulting from the reaction closes the pores and thus increases the corrosion resistance. Parallel to this, chemical sealing methods also exist [15]. In the field of micro- and nanotechnology, the open pore structure is often preferred and sealing is not used here.
Despite the long history of anodizing, there are still existing problems, above all the so-called "burning" and powdery deposits [16, 17]. The phenomenon of "scorching" is caused by uneven growth of the barier layer and the formation of acicular aluminum. These needles extend through the formed oxide layer and thus cause locally very high current densities during anodization. This is associated with a locally very high temperature rise. Powdery layers are created by chemical dissolution of the pore walls if the anodizing times are too long. This results in larger pore diameters, especially at the mouth of the pores, which ultimately lead to wall breakthroughs. The pores begin to grow into each other, resulting in a powdery, softer deposit. Preventing scorching and powdery deposits sometimes requires exactly opposite measures. This is where direct current processes soon reach their limits [17].
The decisive phenomenon behind pulse anodization is the so-called recovery effect (see Fig. 4) [18, 19].
Anodization initially takes place at the potential E1 over a certain period of time (a to b in Fig. 4). After this time (b to c), an equilibrium current is formed. The film formed at potential E1 has a large pore diameter and the underlying barrier layer is thick. If the potential is rapidly lowered to E2, the flowing current also drops sharply (d to e), only to rise again slowly to a constant value (f to g). When the lower potential E2 is reached, layer growth almost comes to a standstill. The thickness of the barrier layer decreases as a result of chemical dissolution. At a certain point, the barrier layer becomes so thin that the electric field begins to recover and allows further film growth. The pores formed at potential E2 have a much smaller diameter than under potential conditions E1. Repetition of this pulse sequence leads to a structure of alternating layers with small and large pore diameters [13].
![Abb. 4: Der während der Pulsanodisierung beobachtbare Erholungseffekt zwischen dem höheren Potential (E1) und dem niedrigeren Potential (E2). Die Erholungszeit wird dabei mit τrec. angegeben [20] Abb. 4: Der während der Pulsanodisierung beobachtbare Erholungseffekt zwischen dem höheren Potential (E1) und dem niedrigeren Potential (E2). Die Erholungszeit wird dabei mit τrec. angegeben [20]](/images/stories/Abo-2022-05/gt-2022-05-0028.jpg) Fig. 4: The recovery effect observable during pulse anodization between the higher potential (E1) and the lower potential (E2). The recovery time is given as τrec[20]
Fig. 4: The recovery effect observable during pulse anodization between the higher potential (E1) and the lower potential (E2). The recovery time is given as τrec[20]
Potential E1 therefore corresponds to rapid layer growth and the formation of hard oxide layers. DC anodization at this potential leads to "burnt" layers over a certain period of time, which can be avoided by switching to a lower potential. As described above, the pulse with the lower potential ensures the thinning of the barrier layer and its more uniform formation [20, 21]. This minimizes the risk of the formation of needle-shaped deposits and improves the current distribution across the individual pores. In addition, thermal management in the layer is improved, as the heat of reaction generated has sufficient time to dissipate and the temperature distribution across the component is much more uniform. This prevents the layers from burning. The layers produced using current pulses are therefore harder, more uniform and in fact show no tendency to burn or form powdery deposits. The frequencies used in pulse anodizing are significantly higher than those used in pulse deposition and are usually in the range of several seconds. Practical reports show improved sealing and better corrosion resistance of such oxide layers on aluminum alloys [22, 23].
Pulse anodizing is used industrially for the surface treatment of aluminium (alloys) and tantalum for the manufacture of electrical capacitors. It has also proven to be advantageous for hard anodizing in many cases. While direct current or alternating current is usually used for anodizing in classic areas such as anodizing, pulse anodizing is well established in the production of microsystems or nanoscale templates. In the production of nanostructures (nanowires and metallic nanotubes), pulse processes are essential for producing the necessary templates with a homogeneous pore structure [11-13]. The pore structure in DC anodizing is far too uneven in terms of the distribution of diameters across the surface to be used for the reproducible production of such nanodevices. In addition, direct current anodizing tends to form branches and defects. Pulse anodizing not only leads to a very uniform and largely defect-free pore distribution, but also enables the pore geometry to be specifically adjusted by selecting the pulse conditions (potentials).
Pulsed Plasma Electrolytic Oxidation (PEO)
Plasma electrolytic oxidation (Fig. 5) is a powerful process for the treatment of light metal surfaces and is particularly suitable for the surface treatment of industrially relevant light metal alloys, especially alloys of aluminum, magnesium and titanium. [24-26] At the start of the process, a passive surface is generated by anodic oxidation. The subsequent application of higher potentials results in the formation of electrical microdischarges between the substrate (workpiece) and the electrolyte used. At the starting point of the arc, a reaction occurs between the substrate and the electrolyte, which leads to local melting of the substrate in this area, the incorporation of electrolyte components and the formation of a ceramic layer. The resulting layer is much more insulating than the initially generated passive layer on the workpiece surface, which leads to the formation of the next arc at a different location. This process is repeated until the entire surface of the workpiece has been completely covered with the ceramic layer. The greatest advantage of PEO coatings is the high coating hardness and therefore good abrasion resistance. A complete surface coating also offers protection of the workpiece material against chemical attack, e.g. from corrosive media.
![Abb. 5: Die einzelnen Schritte eines PEO Prozesses [24] Abb. 5: Die einzelnen Schritte eines PEO Prozesses [24]](/images/stories/Abo-2022-05/gt-2022-05-0029.jpg) Fig. 5: The individual steps of a PEO process [24]
Fig. 5: The individual steps of a PEO process [24]
However, if the process of conversion to an oxide layer is insufficient, pores may form, preferably at the boundaries of the overlapping punctual ceramic surfaces. This allows corrosive (liquid) media to penetrate the light metal surface, which can lead to corrosion reactions. The complete homogeneity of the surface coating, excluding the formation of pores, is therefore of the utmost importance for the protective properties of the oxide layer formed.
The process of plasma electrolytic oxidation is fundamentally based on the use of dilute solutions of non-toxic ions (such as silicates, aluminates, phosphates) as electrolytes, which makes it a particularly environmentally friendly method of surface treatment. The disadvantageous aspect is the relatively high energy requirement, which is considerably higher than in classic galvanic processes. This is due to the comparatively high potentials applied in the range between 400 V and 500 V (in some cases even higher). This is associated with correspondingly powerful rectifier systems and cooling systems and correspondingly high investment costs when scaling up to an industrial scale. The process temperature is a critical factor and has an impact on the quality of the layers produced. For this reason, an (uncontrolled) rise in temperature during plasma electrolytic oxidation should be avoided at all costs.
In addition to the electrolyte composition and the electrolyte temperature, the applied current in particular has a decisive influence on the properties of the oxide layer formed. If the conversion into the ceramic oxide layer is incomplete, pores form in the insulator layer, which are detrimental to the protective effect. Corrosive media reach the substrate surface through the pores and, in the worst case, cause pitting corrosion. The problem of porous PEO layers was recognized early on, but no remedy could be found using direct current processes. The early applications of such coatings were therefore exclusively in the field of wear protection coatings.
At the same time, there were repeated attempts to use them, which initially failed due to the inadequacies of the existing pulse rectifiers. With the increasing performance of high-performance electronics and their introduction into pulse rectifiers, there was a broad switch from direct current technology to pulsed currents for the production of PEO coatings [27-29].
The use of alternating current or bipolar pulses leads to a change in the local solubilities due to a local pH value increase in the cathodic sub-step. The resulting denser layers are accompanied by improved corrosion stability. An increase in the pulse frequency reduces the local energy input due to the associated reduction in the discharge time, which leads to a denser layer with reduced roughness. The use of trapezoidal pulses instead of rectangular pulses eliminates potentially damaging current peaks at the switching points and reduces the formation of pores. By changing the pulse conditions, not only can the physical properties be improved, but the appearance of the coatings can also be influenced. In combination with the electrolyte composition, the color of the coatings can be varied from anthracite-black through all shades of gray to a bright white.
In summary, pulse methods are not only established in the field of PEO coatings, they have become essential. Only the introduction of pulsed current control has made it possible to extend the range of applications from pure abrasion-resistant coatings or porous adhesion promoters before a polymer coating to dense, decorative and corrosion-resistant coatings.
Electrochemical Machining
Electrochemical machining (ECM) is a method for very localized material removal. It is widely used in the precision machining of conductive materials (metals, metal alloys). The workpiece is positioned in the immediate vicinity of the anode (gap width < 1 mm), whereby the shape of the anode is decisive for the machined area. The gap is surrounded by electrolyte solution.
If a current or potential is applied to this arrangement, the workpiece is dissolved directly in the vicinity of the counter electrode. As the dissolution progresses, the counter electrode is precisely tracked to ensure a constant gap size. In this way, the shape of the counter electrode is transferred directly to the workpiece. It is important that the gap is well rinsed in order to remove degradation and by-products and to ensure that the solubility limit of the electrolyte in the gap is not exceeded. In addition, this continuous electrolyte exchange also ensures good dissipation of the heat of reaction generated. A schematic representation of such an arrangement is shown in Figure 6. The process has significant advantages over mechanical methods, including the ability to process hard-to-machine carbides such as tungsten or refractory metals [30-32].
![Abb. 6: Schematische Darstellung des Arbeitsprinzips des elektrochemischen Machinings. Das zu bearbeitende Werkstück (Workpiece) wird in geringem Abstand zur Anode (Tool) platziert. Der Elektrolyt wird in den Spalt zwischen den Elektroden gepumpt. Durch Anlegen eines entsprechenden Stroms wird das Werkstück lokal im Bereich der Anode aufgelöst, wobei durch Nachrücken der Gegenelektrode (Tool Feed) die Größe des Spalts beibehalten wird [1] Abb. 6: Schematische Darstellung des Arbeitsprinzips des elektrochemischen Machinings. Das zu bearbeitende Werkstück (Workpiece) wird in geringem Abstand zur Anode (Tool) platziert. Der Elektrolyt wird in den Spalt zwischen den Elektroden gepumpt. Durch Anlegen eines entsprechenden Stroms wird das Werkstück lokal im Bereich der Anode aufgelöst, wobei durch Nachrücken der Gegenelektrode (Tool Feed) die Größe des Spalts beibehalten wird [1]](/images/stories/Abo-2022-05/gt-2022-05-0030.jpg) Fig. 6: Schematic representation of the working principle of electrochemical machining. The workpiece to be machined is placed a short distance from the anode (tool). The electrolyte is pumped into the gap between the electrodes. By applying an appropriate current, the workpiece is dissolved locally in the area of the anode, whereby the size of the gap is maintained by moving the counter electrode (tool feed) forward [1]
Fig. 6: Schematic representation of the working principle of electrochemical machining. The workpiece to be machined is placed a short distance from the anode (tool). The electrolyte is pumped into the gap between the electrodes. By applying an appropriate current, the workpiece is dissolved locally in the area of the anode, whereby the size of the gap is maintained by moving the counter electrode (tool feed) forward [1]
Neutral salt solutions are usually used as electrolytes and dissolution often takes place in the transpassive range (with oxygen development). However, passivating electrolytes such as NaClO3 or NaNO3 are now often preferred as they lead to more precise results. The side reaction of oxygen evolution reduces the current yield, degradation products adhering to the cathode reduce the efficiency of the process and can sometimes lead to a (geometrically) less accurate resolution. Any deposits of poorly soluble degradation products on the anode (the workpiece) would counteract the electrochemical removal and must therefore be removed from the gap immediately. Deposits in the gap can lead to short circuits or increased heat input (up to boiling electrolytes). In general, this purely electrochemical machining process is very fast, with process times usually ranging from seconds to a few minutes [30, 31].
Electrochemical machining is classically carried out under direct current conditions. However, there is hardly any process in which pulsed electrochemical processes show such a clear advantage over direct current.
Significant advantages of pulsed electrochemical machining are, as proven by a large number of studies and practical reports, an increase in machining accuracy, the removal of reaction by-products and improved thermal management.
First and foremost, the current distribution across the workpiece is of great importance for the precision of machining. This can be achieved by reducing the gap width. Pulse ECM systems stabilize the process with smaller gap widths, particularly via the pulse pause, which allows regeneration of the electrolyte in the gap and temperature equalization. The process can also be controlled via a targeted selection of the applied process parameters (such as the choice of the dominant current density distribution).
The removal of by-products and the possibility of heat equalization are probably the greatest advantages of pulsed current control [33]. In the simplest case, a pulse pause is sufficient to remove adhering gas, poorly soluble reaction products or simply metal ions. The choice of the length of the pulse pause depends on the one hand on the by-products formed and on the other hand on the exchange rate of the electrolyte in the gap. As the electrolyte is kept at a constant temperature, the pulse pause also has a cooling effect (removal of the reaction heat). The effect of the pulse pause can be supported by retracting the tool (or the counter electrode) during the pulse pause and thus enlarging the gap [1, 33]. There are mechanical limits here; in this case, the pulse pauses must be in the range of seconds. In such an approach, the time-out can also be used to correct the position and readjust the gap [1, 33]. If the introduction of a pulse pause is not sufficient to sufficiently clean the electrode surface of by-products, the effect can be enhanced by reversing the polarity of the workpiece. This makes sense, for example, with poorly soluble by-products, as the polarity reversal de facto "blasts off" the reaction products. In this case, the choice of parameters is particularly important in order to prevent an excessive amount of hydrogen being produced on the workpiece surface during polarity reversal.
Electrochemical machining can also be used to produce very fine structures on the order of micrometers [34, 35]. Here, a metallized wafer plate is usually used as the workpiece, which is selectively covered with an insulating polymer mask (photoresist). The general structure is again similar to the general set-up (see above). The anodic dissolution takes place in those areas of the workpiece which are exposed and not covered by the mask. Such etching processes are also known on a purely chemical basis, but the prevailing isotropy of the etching process quickly leads to an infiltration of the masking and thus to inaccurate results. The electrochemical process, on the other hand, exhibits a pronounced anisotropy and the structures are imaged very precisely. The anisotropy is further enhanced by the use of pulsed current, and any passivation effects that may occur are counteracted by reverse pulses.
The reverse procedure is also known. In this case, the counter electrode is masked and the unmasked workpiece is processed [36]. In this setup, the width of the gap plays an even more important role; it should be less than 0.5 mm. Good results with steep etching edges could only be achieved by using current pulses in a rather poorly conductive electrolyte medium.
Surface post-processing of 3D-printed metal components
Post-processing is still one of the biggest challenges in the industrialization of metal 3D printing. The manual mechanical processing steps that are currently mostly used are not suitable for large-scale production in terms of quality assurance and scalability, and undercuts of complex components or interior spaces can only be treated using wet chemical/electrochemical processes. Starting from a CAD file, post-processing is only rarely taken into account when transforming into a print file. The decisive factor is that not a single 3D-printed component can be transferred to (industrial) use directly after printing. The surface roughness after printing is usually far above technically usable values of Ra > 100 µm. Support structures required for printing have to be removed from the component, as do adhering powder residues. To make matters worse, the orientation of the workpiece has a strong influence on the surface roughness. There are major differences between the top side of the printed component and the underside (up-skin - down-skin effects), with the latter surfaces being significantly rougher.
An initial approach to finishing such components was classic (thermodynamically controlled) electropolishing. However, the roughness present after printing is too great for direct electropolishing and must therefore first be reduced using other methods such as mechanical processing or blasting processes. The above-mentioned support structures must also first be removed mechanically. This means that the only remaining processing step for electropolishing is fine leveling, i.e. polishing. This can be classic electropolishing in aqueous media or electropolishing in conductive polymer beads (DLyte process). The surfaces are smoothed by these processes from a moderate initial level of Ra of around 2 µm to a final roughness of usually 0.3 µm. This is hindered by phenomena caused by the pressure and the resulting material structure. The build-up of the workpiece from the powder, line by line, results in a specific waviness. This waviness is not recognized as roughness under thermodynamic control and is therefore not removed. The result is a smooth "hilly landscape" on the surface which can sometimes be problematic for technical use. However, this result cannot be further improved using thermodynamic (direct current) methods. Only the combination with mechanical processes such as vibratory grinding before or after electropolishing then also provides macroscopically smooth surfaces.
Here too, pulsed electrochemistry can overcome existing limitations. Especially when removing support structures, pulsed current conduction is a powerful tool for dissolution due to the possibility of influencing the potential field and the field line distribution on the component.
The most prominent representative of dynamic electrochemical post-processing is shearing. Figure 7 shows the schematic structure of the shearing process. Similar to electropolishing, the component is conductively contacted and connected as an anode. By applying a pulse current and sometimes in combination with a chemical attack via the selection of specially adapted process media, support structures and powder residues are removed and the surface is leveled.
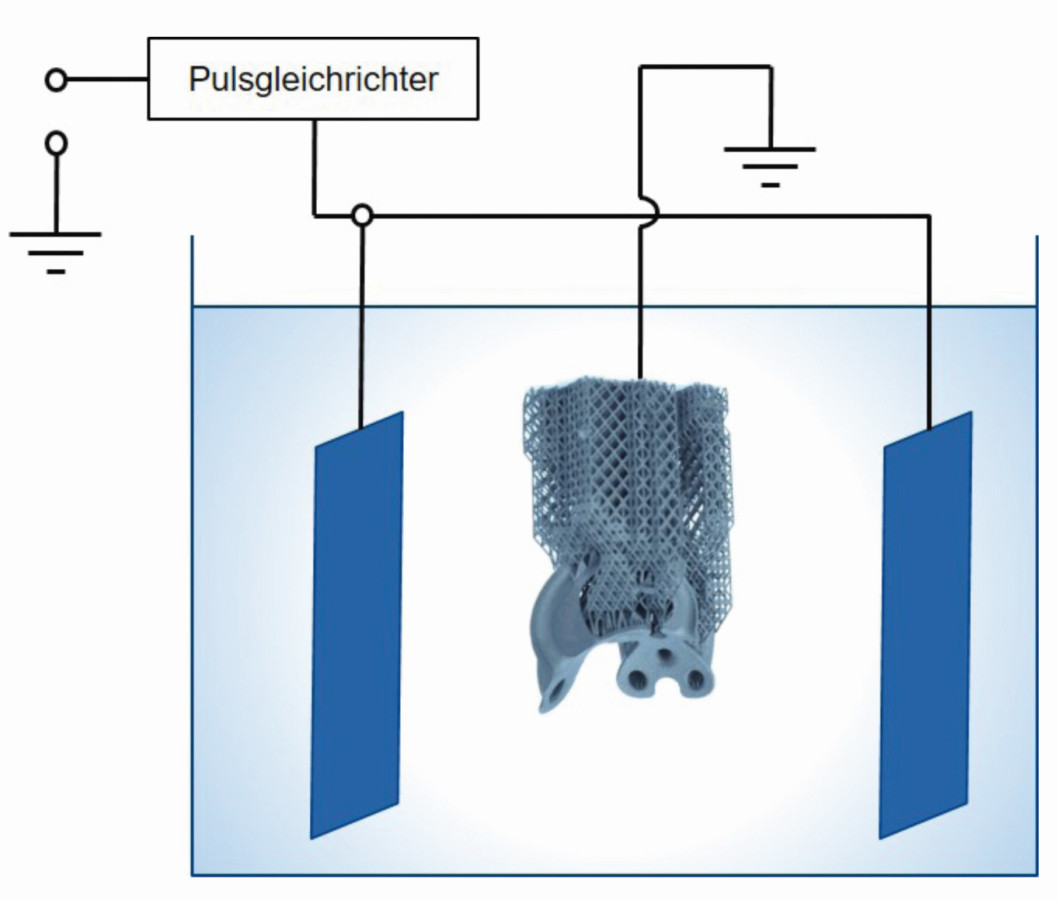 Fig. 7: Schematic structure of the shearing process. Similar to electropolishing, the component is conductively contacted and connected as an anode. Support structures and powder residues are removed and the surface is leveled by applying a pulsed current and sometimes in combination with a chemical attack via the selection of specially coordinated process media
Fig. 7: Schematic structure of the shearing process. Similar to electropolishing, the component is conductively contacted and connected as an anode. Support structures and powder residues are removed and the surface is leveled by applying a pulsed current and sometimes in combination with a chemical attack via the selection of specially coordinated process media
Supported by pulsed current and combined with chemical dissolution, this process can also be used to specifically dissolve internal support structures. In addition to the removal of these support structures and the removal of powder residues, the surface of the component is also leveled to an industrially acceptable level [37-39]. Without the use of pulsed current, this process would be inconceivable and would be subject to the same limitations as DC electropolishing. Due to the possibility of processing geometrically difficult to access areas and interior spaces of the components, this chemical-electrochemical process acts as an enabler of new component geometries and thus supports design freedom, which is one of the most important strengths of 3D printing.
Summary of the process
Electrochemical processes guided by pulsed current are only slowly developing from niche areas into general electroplating technology. Within niches and for very specific applications, however, they have become an integral part of industrial production and act as an enabler for the production of complex components in many areas.
At the beginning of the introduction of pulsed electrochemical processes, the focus was on pulse deposition, but today there are a multitude of applications. Pulse processes have become particularly well established in the anodic processing of metallic components. The control of anodic dissolution or passivation using pulse processes offers a number of advantages over DC processes. Modern rectifier technology is no longer a limitation and is able to support pulsed processes as required. Contrary to what was originally assumed, with pulse deposition playing a subordinate role, pulsed electrochemical processes are now an important mainstay of electrochemical surface technology.
Literature
[1] W.E.G. Hansal; S Roy: Pulse Plating, Eugen G. Leuze Verlag, Bad Saulgau, F.R.G., 2012
[2] D. Landolt: Electrochimica Acta, 248 (2017) 75
[3] M. Datta: IBM J. Res. Develop, 37 (1993) 207
[4] M. Buhler;, A. Rettinghaus; T. Hinte; A. Visser: Journal PCMI, 95 (2004), 5
[5] W.E.G. Hansal, Eurofinish, Bremen, 2009
[6] W.E.G. Hansal: Schriftenreihe Werkstoffe und werkstofftechnische Anwendungen, (ed. T. Lampke) 2018, 80
[7] A. Bhuyan; B. Gregory; H. Lei; S.Y. Yee; Y.B. Gianchandani: IEEE Sensors, 2005, 314
[8] N. Eliaz; O. Nissan; J. Biomed. Mater. Res, 83A (2007), 546
[9] A. Lozano-Morales; M. Inman; E.J. Taylor: Plat. Surf. Fin., 96 (2009), 5
[10] H. Kaesche: Corrosion of Metals: Physiochemical Principles and Current Problems, Springer, Berlin, 2003
[11] M. Madou: Fundamentals of Microfabrication, 2nd Edition, CRC Press, 2002
[12] G.D. Sulka in Nanostructured Materials in Electrochemistry (Editor A. Eftekhar), Wiley-VCH, 2008
[13] W. Lee: J. Electron. Mater, 62 (2010), 57
[14] D. Landolt: Corrosion and Surface Chemistry of Metals, EPFL Press, Lausanne, 2007
[15] J.W. Diggle; T.C. Downie; C.W. Goulding: Chemical Reviews, 69 (1969), 365
[16] P.G. Sheasby; R. Pinner: The Surface Treatment and Finishing of Aluminum and its Alloys, 6th Edition, ASM International, 2001
[17] A.D Juhl: Pulse Anodising of Extruded and Cast Aluminium Alloys, PhD Thesis,Technical University of Denmark, 1999
[18] J.F. Murphy; C.E. Michelson: Conference on Anodising, Nottingham, 1961
[19] H. Takahashi:;M. Nagayama; H. Akahori; A. Kitahara: J. Electron. Microscopy, 22 (1973), 149
[20] K. Yokoyama; H. Konno; H. Takahashi; M. Nagayama: Proceedings of 2nd International Symposium on Pulse Plating, 1982
[21] K. Yokoyama; H. Konno; H. Takahashi; M. Nagayama: Plat. Surf. Fin., 7 (1982), 62
[22] C. Colombini: Finishing, 12 (1988), 34
[23] C. Colombini: Metal Finishing, 90 (1992), 31
[24] R. Mann; W.E.G. Hansal; S. Hansal: Transactions of the IMF, 92 (2014), 297-304
[25] N. Godja; N. Kiss; C. Löcker; A. Schindel; A. Gavrilovic; J. Wosik; R. Mann; J. Wendrinsky; A. Merstallinger; G.E. Nauer: Tribology International, 43 (2010), 1253-1261
[26] N. Godja; W.E.G. Hansal; R. Mann; C. Kleber; S. Hansal: Transactions of the IMF, 91 (2013), 321-329
[27] 46. P. Shashkow; G. Khomutov; A. Yerokhin; S. Usov: Patent PCT/GB2012/050268, 2012
[28] 47. R. Mann;, W.E.G. Hansal; S. Hansal: Patent PCT/AT2016/050188, 2016
[29] A.L. Yerokhin; X. Nie; A. Leyland; A. Matthews; S.J. Dowey: Surf. Coat. Technol., 122 (1999), 73
[30] A.D. Davydov; V.M. Volgin; V.V. Lyubimov: Russ. J. Electrochem., 40 (2004), 1438
[31] C. van Osenbruggen; C. de Regt: Philip Tech. Rev., 42 (1985), 22
[32] K.P. Rajurkar; D. Zhu; J.A. McGeough; J. Kozak; A. De Silva: Annals of the CIRP, 48 (1999), 567
[33] J. Kozak; K.P Rajurkar; B. Wei: J. Eng. for Industry, 116 (1994), 316
[34] M. Datta; R.V. Shenoy; L.T. Romankiw: J. Eng. for Industry, 118 (1996), 29
[35] M. Datta: IBM J. Res. Develop, 42 (1998) , 563
[36] I. Schönenberger; S. Roy: Electrochim. Acta, 51 (2005), 809
[37] W.E.G. Hansal: Jahrbuch Oberflächentechnik, (ed. T. Sörgel) 2019, 75, 48
[38] W.E.G. Hansal: X-Technik Additive Fertigung, 2 (2019), 12
[39] W.E.G. Hansal: X-Technik Additive Fertigung, 4 (2019), 24


