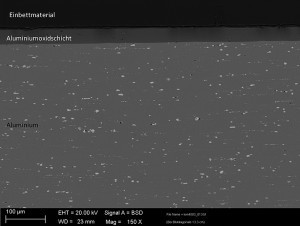
The sample plates of EN AW 2024 and the Alumold® 350/Tempral cast plate EN AW 2219 were anodized in a sulphuric acid electrolyte with direct current at room temperature. The deposition rates were 4-6µm per minute, with current densities of 7-10A/dm2. An optimized and specially designed system technology enables a uniform transport of heat away from the component without additional cooling in the process bath. This is the only way to prevent "burns" on the component due to increased re-dissolution. The sample panels do not exhibit any negative edge effects or "burns". A uniform layer thickness distribution with layer thicknesses of 40-45 µm was achieved.
The aluminum oxide layers were examined using cross-sections on a scanning electron microscope (SEM) and the phase structure was determined with the aid of EDX spectroscopy (Energy Dispersive X-Ray Spectroscopy).
Results - Layer characterization EN AW 2024
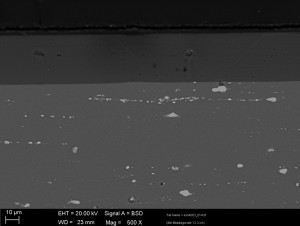
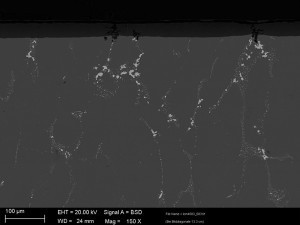
The aluminum oxide layer thickness is uniform and shows no significant defects in the aluminum oxide layer (see Fig. 2 and 3).
The phase incorporation is more clearly recognizable in Figure 3. The intermetallic phases of the 2024 alloy are largely oxidized and the non-oxidizable portion is incorporated into the oxide layer.
This mechanism disturbs the structure of the aluminum oxide layer only very little. Due to the thicker oxide layers, the influence of the phase size is also significantly lower. No negative re-dissolution mechanisms of the oxide layers are recognizable.
Note: The visible dark gap above the oxide layer is due to the sample preparation.
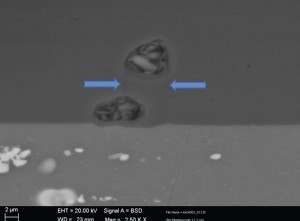
Figure 4 shows the distribution and reaction of the intermetallic phases of the alloy. The alloy phases were integrated into the layer structure (light phase areas) and partial areas of the phases (dark phase areas) were oxidized. In this image, the dark grey fine lines (see marking) clearly show how the copper-containing phases were displaced during the oxide layer build-up. As oxide layers grow out of the aluminium during anodization (anodizing process), phases in the oxide layer may shift during the growth phase (process phase).
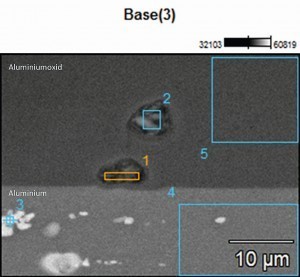
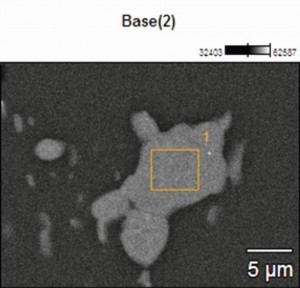
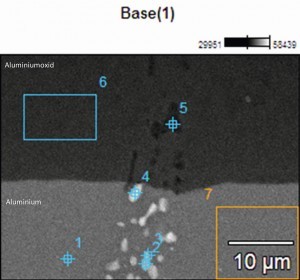
In order to obtain an overview of the phase distribution and composition, the phases were analysed and characterized using EDX (Energy Dispersive X-Ray Spectroscopy) on the SEM (scanning electron microscope). The compositions of the investigated areas are summarized in Table 3. Measuring points 1 and 2 were placed directly on the phases incorporated in the aluminum oxide layer. A high proportion of oxygen (O) and aluminum (Al) can be seen, which indicates oxidation of the phase and the presence of aluminum oxide. In addition, proportions of silicon (Si) and copper (Cu) as well as a small proportion of magnesium (Mg) can also be detected, which in turn indicates the integration of some intermetallic components. The sulphur content (S) is due to the anodization process in sulphuric acid.
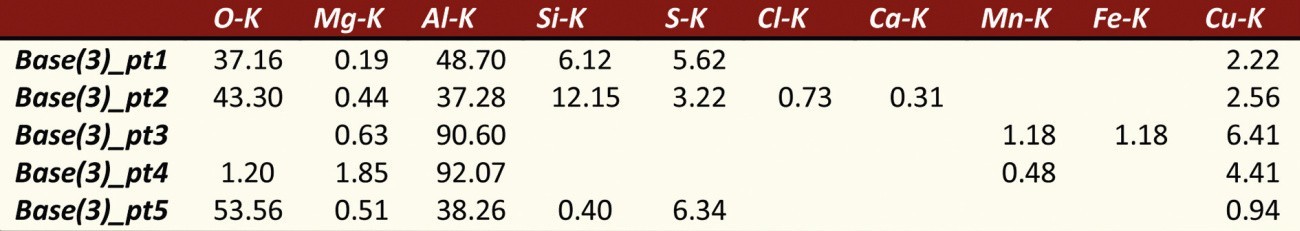 Tab. 3: Composition of the phases - EN AW 2024 (see Fig. 5)
Tab. 3: Composition of the phases - EN AW 2024 (see Fig. 5)
Measuring point 3 characterizes the phase in the aluminium base material and has a high copper content. The shape and size are comparable to the phases from measuring points 1 and 2.
Results - Layer characterization EN AW 2219
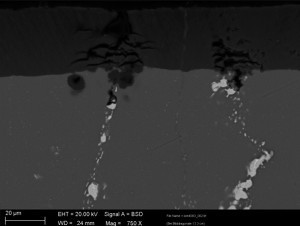
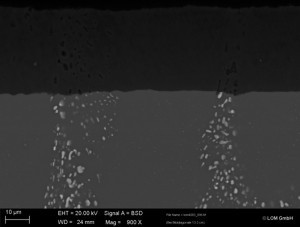
Although the aluminum oxide layer thickness is uniform, it exhibits significant defects caused by the intermetallic phases in the aluminum oxide layer (see Figs. 6, 7 and 9).
The material structure of the cast plate EN AW 2219 differs significantly from a rolled plate structure. The phases in the material structure are very large. The structure of the oxide layer is significantly impaired. However, it was possible to demonstrate that high layer thicknesses can also be achieved on alloys with a very high copper content using optimized plant technology.
Figures 7 and 9 show even more clearly the influence of phase incorporation. The copper-containing alloy phases of the 2219 alloy are completely dissolved. Due to their size and shape, they cause pronounced oxide layer defects. The oxide layer shows cracks and in some cases the defects in the layer extend to the base material. During anodization, electrolyte can run into these cracks and can lead to oxidation along the phase chain into the base material. The oxide layers are greatly weakened by the relatively large holes and cracks in the oxide layer. Corrosive media can penetrate deep into the oxide layer down to the base material, infiltrate layer systems and attack the base material. Mechanical stability is also not guaranteed. Mechanical loads can cause the oxide layer to break off.
 Tab. 4: Composition of the phase - EN AW 2219 (see Fig. 8)
Tab. 4: Composition of the phase - EN AW 2219 (see Fig. 8)
Conclusion:
Aluminium materials with a high copper content are difficult to anodize using conventional standard processes. Often only layer thicknesses of up to 10-15 µm can be achieved. This is often too low for high demands such as corrosion resistance or abrasion. Functional surface protection is required for optimum use of high-alloy materials. Optimized aluminium oxide coatings are necessary to open up new fields of application in the area of high-strength aluminium alloys. Through the targeted removal of process heat from the component during the anodization process, oxide layer thicknesses of 40 µm and higher can be achieved on high-copper and high-strength aluminium materials. Additional cooling in the process bath is not necessary. A clever combination of system design and process parameters enables us to achieve high deposition rates of aluminum oxide in a short time. This combination prevents partial overheating of the component. With the optimized system technology, deposition rates of 4 to 6 µm/min can be achieved. Short process times and no cooling capacity in the process bath also make the anodization process energy-efficient.
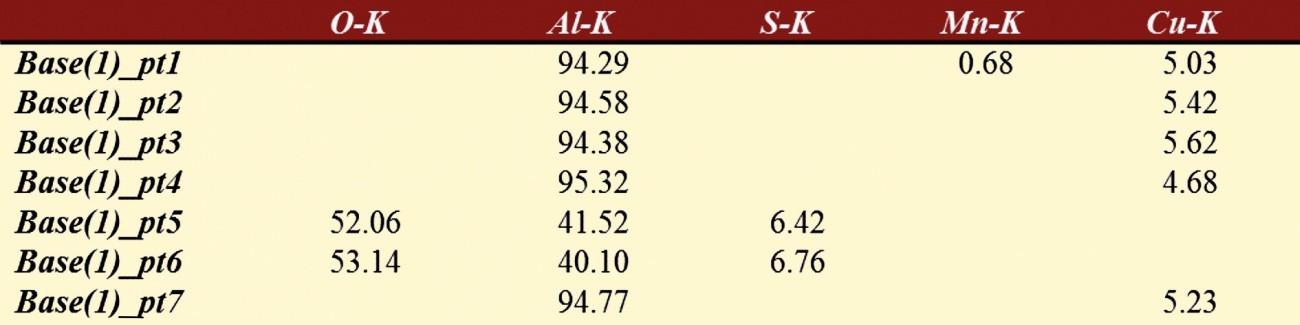 Tab. 5: Composition of the phases - EN AW 2219 (see Fig. 10)
Tab. 5: Composition of the phases - EN AW 2219 (see Fig. 10)
In the evaluation of the SEM images and EDX analyses, EN AW 2024 showed a uniform oxide layer thickness of 40-45 µm and a promising incorporation of the intermetallic phases in the oxide layer.
The 2219 aluminium alloy showed a high amount of large alloy phases with a tendency to be completely dissolved during the anodizing process. The result is a marked layer defect, which in some cases extends to the base material.
It is worth thinking further in this direction and, above all, pursuing EN AW 2024.
We'll stay tuned ...