Anodizing aluminium alloys with a high copper or silicon content is often very difficult using conventional anodizing processes. The aluminum material group of 2000 series alloys in particular has a high copper content. These alloys in particular have a tendency to "burn" during the anodizing process. The so-called "burning" describes a very strong re-dissolution effect on the component. This effect is supported by partial overheating and stress peaks at component edges. The component is partially attacked and dissolved. In the worst case, this can lead to the complete destruction of a component.
High-alloy aluminum materials or cast products are often used in the automotive, aerospace and mechanical engineering industries. In particular, the aluminum alloys 2000 (main alloying element copper) and 7000 (main alloying element zinc) are frequently used due to their high strength and high-temperature resistant material properties. The weight of a part is often an important factor and can be drastically reduced by using the right aluminum material. However, the high amount of alloying elements in the material also has some disadvantages. EN AW 2024 (AlCu4Mg1), for example, has poor corrosion resistance and it is very difficult to achieve oxide layer thicknesses of more than 10-15 um in standard anodizing processes (sulphuric acid). EN AW 7075 (AlZn5.5MgCu) has good anodizing properties but low corrosion resistance with a tendency to stress corrosion cracking.
Functional surface protection is required for optimum use of the high-alloy materials. Oxide layer thicknesses with good layer quality could open up new fields of application. For this reason, we have launched an internal development project that focuses on the surface treatment of high-alloy aluminum materials and the influence of alloying elements. The aim of the project was to develop an anodization process with high deposition rates and good oxide layer quality under easy-to-handle process conditions. Two materials with a high copper content were selected for the project, EN AW 2024 with a copper content of approx. 4.% and EN AW 2219 with a copper content of approx. 6.%. The 2024 is used very frequently in various areas of the aerospace industry, while the 2219 aluminum alloy is a cast plate material from the manufacturer Constelium and is often used in mold making.
The strategic focus of the development project:
- High deposition rates (4-6 µ/min)
- Short processing time
- Handling at room temperature
- Avoidance of complex process bath compositions
- High oxide layer quality
- Higher oxide layer thicknesses in comparison
- Easy implementation in existing production lines
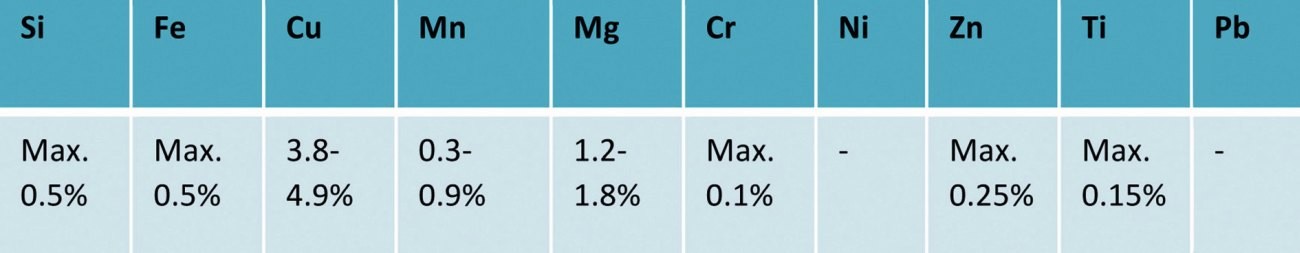 Tab. 1: EN AW 2024 T351 - AlCu4Mg1 - composition
Tab. 1: EN AW 2024 T351 - AlCu4Mg1 - composition
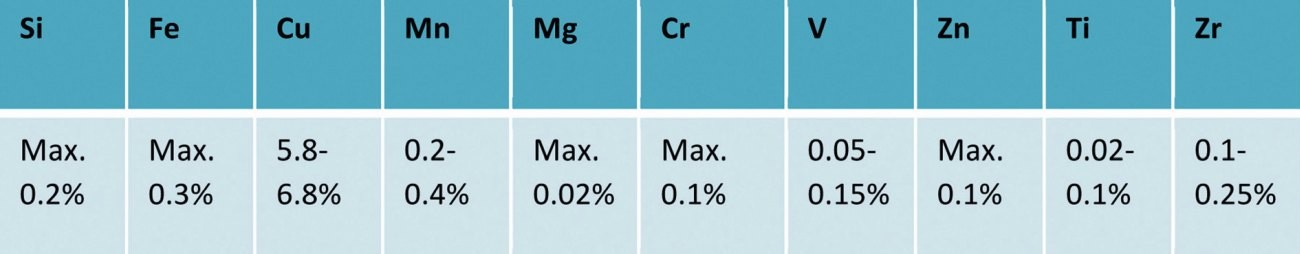 Tab. 2: Alumold® 350/Tempral EN AW 2219 T851 - AlCu6Mn - composition
Tab. 2: Alumold® 350/Tempral EN AW 2219 T851 - AlCu6Mn - composition
Material properties EN AW 2024 T351
- Medium to high strength
- Excellent dimensional stability
- Good mechanical processing
- Low corrosion resistance
- Suitable for high temperature applications
- Not weldable
- Rm 400-440 MPa, A50 7-14 % (depending on sheet thickness)
- Technical anodizing: Poor (limited oxide layer thickness)
Area of application: Aerospace, machine industry, injection molding (plastics industry)
Material properties Alumold® 350/Tempral EN AW 2219
- Poor corrosion resistance
- Suitable for high-temperature applications (service temperatures up to 180 °C)
- Excellent mechanical processability
- Easy to polish (for mold making)
- Good weldability
- Technical anodizing: Poor (limited oxide layer thickness)
Areas of application: Molds for injection molding, composite molding, mold making in general, plastics industry
Influence of the intermetallic phase of the alloys on the coating properties
Three types of interaction of intermetallic phases in oxide layers are recognizable:
Integration, the alloy phase is incorporated into the oxide layer.
The incorporation of alloy phases into the oxide layer has advantages and disadvantages. In order to be incorporated, the phases must be surrounded by the oxide layer. If the phases are very large, this can lead to stresses in the oxide layer and form internal cracks. If the phases are easy to incorporate and no stresses occur in the layer, these only have an influence on properties such as breakdown voltage or corrosion resistance, whereby the position of the phase is important.
Oxidation, the alloy phase is also oxidized during anodization and incorporated into the coating. These phases have very little effect on the oxide layer. The formation of the oxide layer can be slightly delayed, depending on whether the phase is faster or slower to oxidize than the pure aluminium. However, an oxide is also present. Interactions such as galvanic corrosion mechanisms can therefore be ruled out.
Dissolution, the alloy phase is dissolved during the process and holes are created in the oxide layer. The worst case is when the phases are very large. The oxide layers are then not uniform in structure and media, such as salt solutions, can penetrate the oxide layer more easily and weaken it from the inside. The layer structure is also weakened when the layers are subjected to mechanical stress. Oxide layer components can break out. If the phases are very small and evenly distributed, the influence is smaller but should not be underestimated.
Of course, it always depends on the application and the ambient conditions.
Intermetallic phases - some examples:
Si - trend towards integration
Cu (e.g. CuAl2), Mg (e.g. β-AlMg) - tendency towards dissolution and oxidation
Fe (e.g. α-AlFeSi) - tendency towards dissolution and integration.
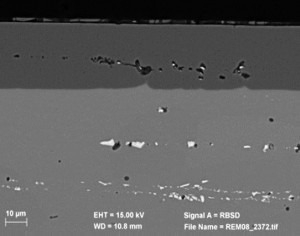 Fig. 1: 800x SEM image of a 6061 aluminum alloy (plate material) in cross-sectionTheimage in Figure 1 shows a 6000 alloy, but explains the mechanisms in the formation of the aluminum oxide layer and the behavior of the intermetallic phases very clearly. The size of the phases and their distribution can be clearly seen in the base material. The cross-section was taken along the direction of rolling, so the lines of the phase chains are clearly visible. The white areas of the phases in the oxide layer indicate an integration of the phase portion. However, phase components were also oxidized or partially dissolved. The layer thickness distribution is uniform and the oxide layer does not show any impairment in the upper area of the aluminum oxide layer in this image. Of course, phase size and shape also depend on the manufacturing process. The phases and phase chains in rolled products in the form of plates and sheets are elongated and have a linear appearance, whereby the shape of the phases in cast materials can be classified as globular.
Fig. 1: 800x SEM image of a 6061 aluminum alloy (plate material) in cross-sectionTheimage in Figure 1 shows a 6000 alloy, but explains the mechanisms in the formation of the aluminum oxide layer and the behavior of the intermetallic phases very clearly. The size of the phases and their distribution can be clearly seen in the base material. The cross-section was taken along the direction of rolling, so the lines of the phase chains are clearly visible. The white areas of the phases in the oxide layer indicate an integration of the phase portion. However, phase components were also oxidized or partially dissolved. The layer thickness distribution is uniform and the oxide layer does not show any impairment in the upper area of the aluminum oxide layer in this image. Of course, phase size and shape also depend on the manufacturing process. The phases and phase chains in rolled products in the form of plates and sheets are elongated and have a linear appearance, whereby the shape of the phases in cast materials can be classified as globular.
The alloys EN AW 2024 and 2219 have a high copper content in the aluminum. They are difficult to anodize and the phase distribution is very pronounced. They influence layer growth and disrupt the layer structure. The aim of the internal development project was to optimize a higher layer thickness on alloys with a high copper content and the influence of the existing alloy phases.
The possibility of depositing higher layer thicknesses on high-copper alloys can have a positive influence on the incorporation of the phases. Although this does not change the type of phase reaction during deposition, thicker oxide layers can cushion negative effects or reactions. The longevity of components can be significantly extended as a result.
-continued-


