Pickling is a fundamental part of component pre-treatment. At the same time, hydrogen embrittlement can occur, which can be counteracted with inhibitors. The new DIN standard 50940 Part 2 is intended to provide a reliable framework for future applications.
 C-ring in HCI pickling with pickling additiveComponent cleaning processes, especially pre-treatment processes, sometimes receive only limited attention in process monitoring in operational practice. This is in contrast to the technical importance of pre-treatment processes. After all, they are responsible for the removal of interfering contamination, adsorption layers, oxide and scale layers or mill scale during the coating processes. Pre-treatment processes ensure optimum adhesion of the coating and thus indirectly influence the application properties of the component.
C-ring in HCI pickling with pickling additiveComponent cleaning processes, especially pre-treatment processes, sometimes receive only limited attention in process monitoring in operational practice. This is in contrast to the technical importance of pre-treatment processes. After all, they are responsible for the removal of interfering contamination, adsorption layers, oxide and scale layers or mill scale during the coating processes. Pre-treatment processes ensure optimum adhesion of the coating and thus indirectly influence the application properties of the component.
In chemical, electrochemical and fusion metallurgical coating processes, the pickling process step within component pre-treatment plays a key role in removing interfering influences from component surfaces prior to component coating. Surface contaminants are usually attacked in pickling processes using inhibited acid baths. Each bath component takes on key tasks. For example, inhibitors counteract unwanted metal dissolution or possible material embrittlement due to hydrogen during the pickling process. Other surface-active substances also enable the targeted removal of surface contamination.
Although much work has been done in the past on the effect and monitoring of pickling additives in pickling processes, it has not yet been possible to develop a national standard that provides a reliable framework for the assessment of pickling processes and their pickling additives.
With the creation of a new DIN standard under the working title "DIN 50940 Determination of the inhibiting effect of pickling inhibitors - Part 2: Methods for the determination of hydrogen evolution in pickling processes", an existing gap in standardization is to be closed.
Status of the working hypotheses on hydrogen embrittlement
According to the current state of knowledge, various hypotheses on material embrittlement due to hydrogen are being discussed. Today, the most common models regarding the mechanism of hydrogen-induced material embrittlement of high-strength steels are the hydrogen enhanced decohesion (HEDE) theory and the hydrogen enhanced localized plasticity (HELP) model [1].
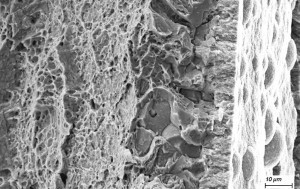
The HEDE model is based on the assumption that the H-induced damage is caused by a reduction in the cohesive forces between the atoms in the metal matrix along crystallographic planes or phase boundaries. The characteristic fracture pattern is a brittle fracture with low energy mainly along the grain boundaries (Fig. 1).
In contrast, the HELP model assumes a reduction in the Peierls* stress due to hydrogen atoms accumulating in the tensile and shear stress range of dislocations, which in turn leads to easier dislocation movement in the presence of external tensile stresses. According to current knowledge, the HELP model is the only experimentally proven damage model [2, 3]. The fractographic fracture surface feature is often a low-energy crack (brittle material behavior) which then often turns into a ductile forced fracture. Here, the honeycomb structures typical of ductile forced fractures can be observed in the fracture surface morphology (see Fig. 2). If both failure theories overlap, the observed mixed fractures exhibit both characteristic features of brittle fractures, e.g. intercrystalline brittle fracture, as well as deformation-rich/energy-rich forced fracture components in the fracture surface.
Status of standardization
As a result of the standards research, a large number of standards were identified that deal directly or indirectly with material embrittlement caused by hydrogen. The following list does not claim to be exhaustive and does not evaluate the state of knowledge formulated in the standards or their contents.
DIN 50969 Part 1-3
Prevention of production-related hydrogen-induced brittle fractures in high-strength steel components - Part 1:2009 Preventive measures
Prevention of production-related hydrogen-induced brittle fractures in high-strength steel components - Part 2:2013 Tests
Avoidance of production-related hydrogen-induced brittle fractures in high-strength steel components - Part 3:2018 Subsequent in-service influences and extended tests
DIN EN ISO 19598:2017
Metallic coatings - Electroplated zinc and zinc alloy coatings on ferrous materials with additional Cr(VI)-free treatments
DIN EN ISO 7539-5:1995
Corrosion of metals and alloys - Stress corrosion cracking test - Part 5: Preparation and use of C-ring specimens
DIN EN ISO 17081:2014
Electrochemical method for the measurement of hydrogen permeation and the determination of hydrogen uptake and transport in metals
DIN EN ISO 4042:2018
Fasteners - Electroplated coating systems
DIN EN ISO 15330:2000
Fasteners - Strain test for the detection of hydrogen embrittlement, method with parallel bearing surfaces
ISO 9587: 2007
Metallic and other inorganic coatings - Pretreatment of iron or steel to reduce the risk of hydrogen embrittlement
ISO 9588: 2007
Metallic and other inorganic coatings - Post coating treatments of iron or steel to reduce the risk of hydrogen embrittlement
ISO 16573-1:2020
Steel - Measurement method for the evaluation of hydrogen embrittlement resistance of high strength steels - Part 1: Constant load test
ISO 16573-2 (in preparation)
Steel - Measurement method for the evaluation of hydrogen embrittlement resistance of high strength steels - Part 2: Slow strain rate test
ASTM F2660:2020
Standard Test Method for Qualifying Coatings for Use on A490 Structural Bolts Relative to Environmental Hydrogen Embrittlement
ASTM 519-17a:2018 (withdrawn)
Standard Test Method for Mechanical Hydrogen Embrittlement. Evaluation of Plating/Coating Processes and Service Environments
ISO/TR 20491:2019
Fasteners - Fundamentals of hydrogen embrittlement in steel fasteners
Status of the measurement methods
The measurement methods available today attempt to capture the system of material embrittlement caused by hydrogen in all its complexity. The interaction of technical influencing variables such as material, phase interface reactions, ambient medium and mechanical load is shown in Figure 3. If a critical state occurs between these influencing parameters, a delayed component failure occurs due to hydrogen-induced material embrittlement within the specified operating situation.
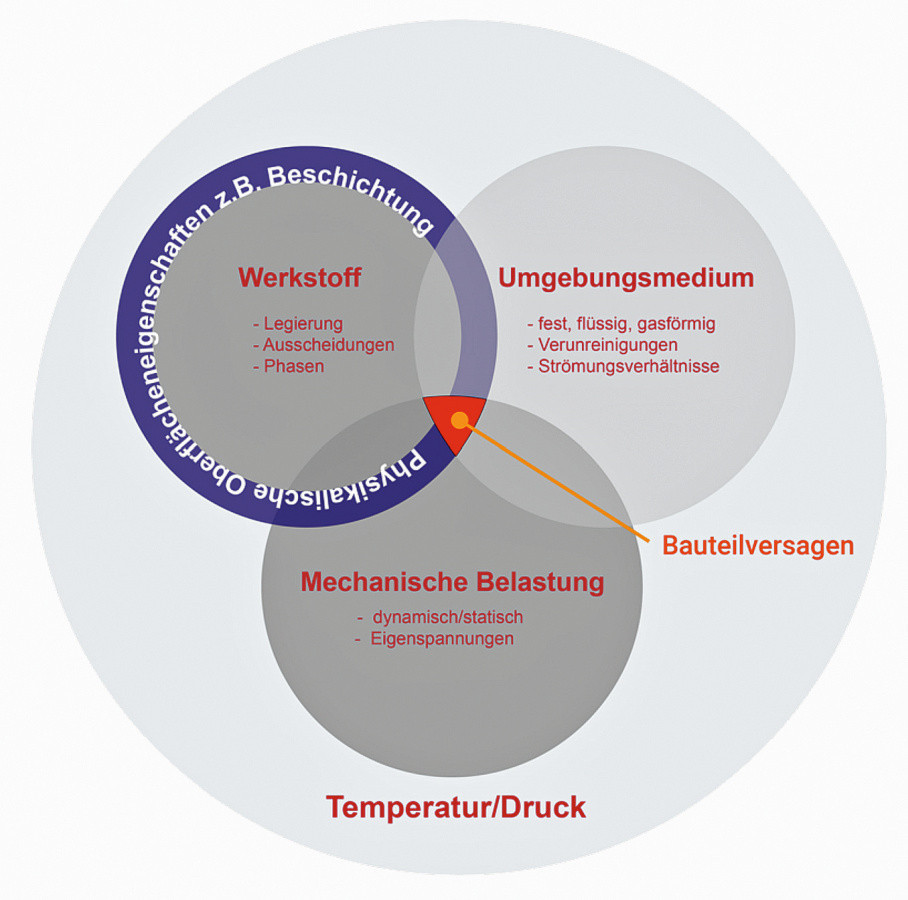 Fig. 3: Technical input parameters for describing material embrittlement caused by hydrogen
Fig. 3: Technical input parameters for describing material embrittlement caused by hydrogen
The challenge was to test today's widely used test methods with regard to their application in operational practice for often highly dynamic pretreatment processes and to evaluate their ease of use in day-to-day operations without losing focus on the development and testing of pickling additives as part of product development.
Due to the dynamic nature of pre-treatment processes, particularly pickling, the overarching aim was to identify a monitoring method that enables in-situ monitoring of pickling processes for material embrittlement caused by hydrogen. As part of the brainstorming and the literature and standards research, various tension tests, measurement methods for determining hydrogen permeation and the measurement method of thermal desorption spectroscopy using mass spectrometry were identified as applicable test methods. These methods were evaluated according to a series of requirement criteria - the results are summarized in Table 1.
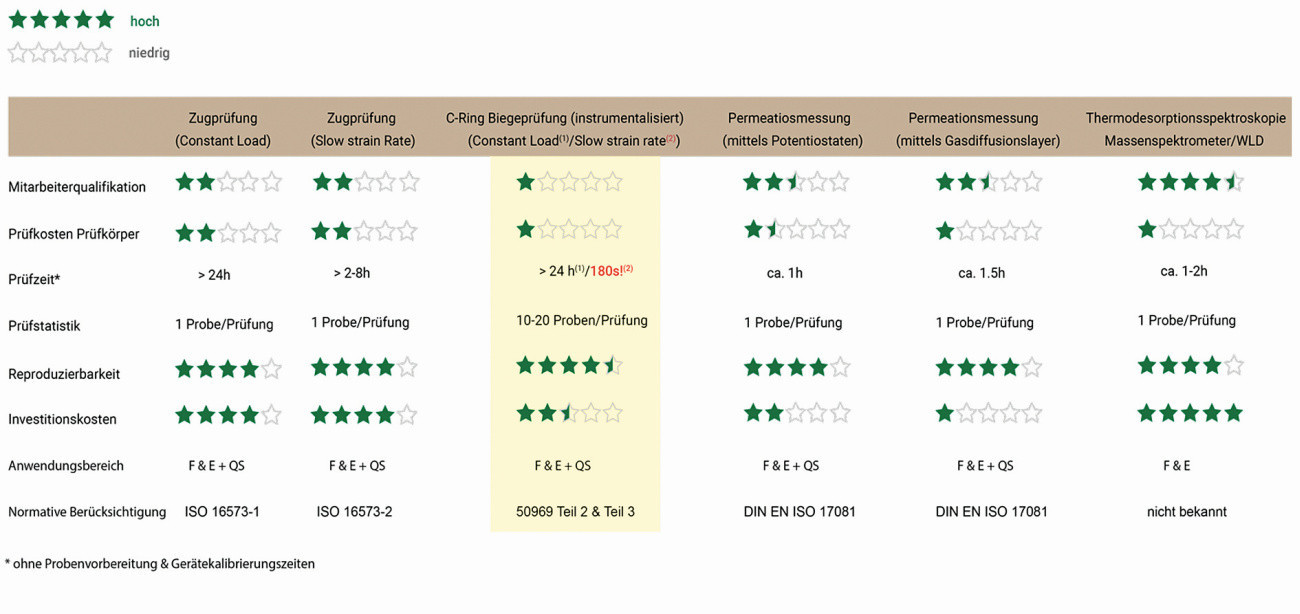 Table 1: Evaluation of the test methods with regard to their handling, quality of information and investment costs
Table 1: Evaluation of the test methods with regard to their handling, quality of information and investment costs
Taking into account the objective of in-situ process monitoring formulated at the beginning with the requirement for reproducible, statistically verified test results, the instrumentalized C-ring test method meets all the requirements. On the basis of the formulated decision criteria, a cross-check is to be carried out to determine whether there is consistency of results between the instrumentalized C-ring bending test, the permeation measurement and the thermal desorption spectroscopy. In preparation for the work on the new standard for the prevention of hydrogen embrittlement in pretreatment processes, a benchmark agreed within the working group is to be used to check whether it is possible to achieve a consistent, valid and holistic measurement result and thus recommend the use of the test methods for further tests or for normative consideration.
The benchmark
Based on the formulation of the objective and the defined decision criteria, a test matrix was agreed, which makes it possible to compare the tension tests, the permeation measurements and the thermodesorption spectroscopy with each other for consistency of results.
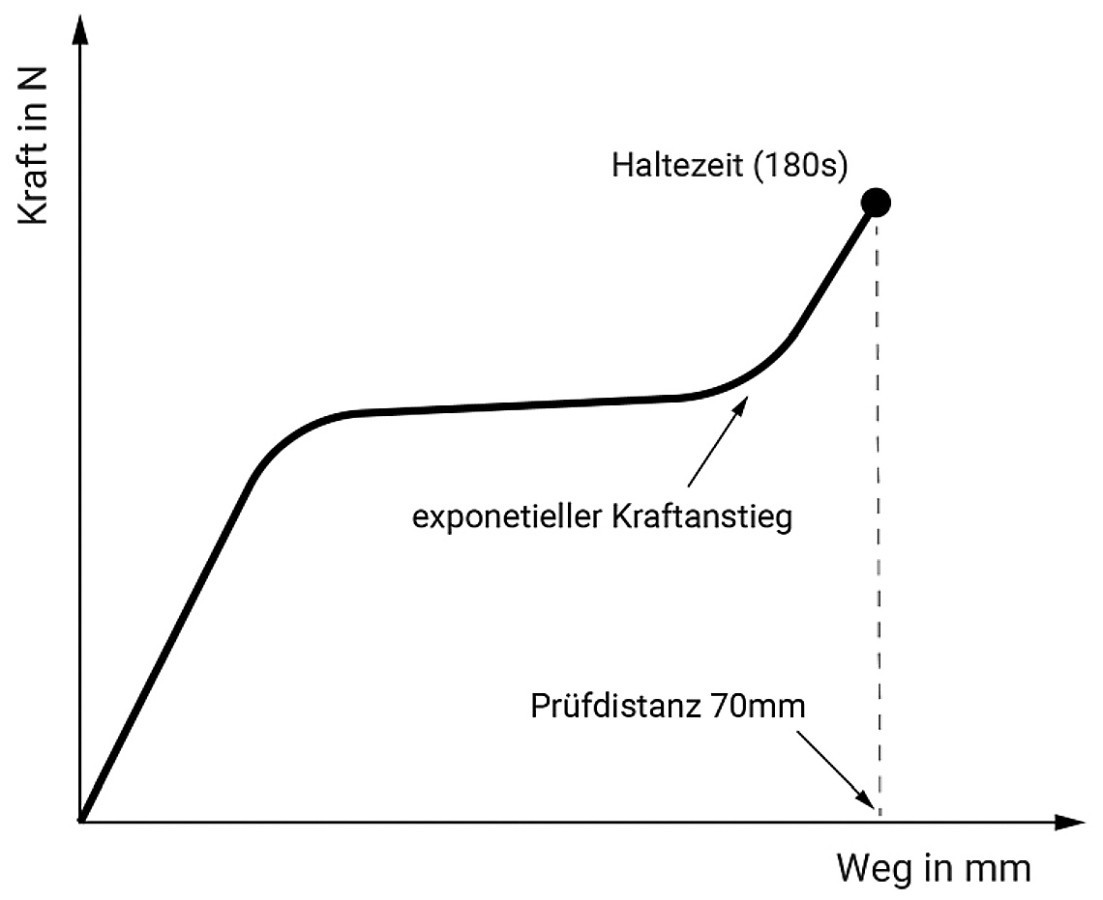 Fig. 5: Schematic test sequence for C-ring tensioning using constant strain rate
Fig. 5: Schematic test sequence for C-ring tensioning using constant strain rate
Instrumentalized tension test on C-ring test specimens and pickling parameters
 Fig. 4: Test station section for the instrumentalized C-ring bending test The instrumentalized tension tests were carried out by the benchmark participants on digitalized test stands with 20 measuring stations (see Fig. 4).
Fig. 4: Test station section for the instrumentalized C-ring bending test The instrumentalized tension tests were carried out by the benchmark participants on digitalized test stands with 20 measuring stations (see Fig. 4).
The reference material C75 in the strength classes 450HV5, 500HV5, 550HV5 and 600HV5 was defined as the basis for the tests. In addition, three commercially available pickling additives were included in the test program, the concentration of which was based on the manufacturer's recommendations. The pickling times of 5 and 15 minutes are based on exposure times in operational pickling processes. The pickling baths are prepared anew before each test in order to exclude the inhibiting influence of dissolved metal ions such as Fe2+/Fe3+.
The mechanical load on the test specimens was applied by means of a constant strain rate ( Fig. 5). The test speed was set at 0.7 mm/s. The test distance of 70 mm results from the diameter of the C-ring specimens of 28 mm. At a test distance of 70 mm, the bending test of the bent, almost stretched specimen changes to a tensile test. Due to the exponential increase in force, the transition from the bending test to a tensile test was formulated as a termination criterion for a stretched length of 80 %.
Table 2 provides an overview of the test program, the test procedure and the pickling parameters of the C-ring tensile tests.
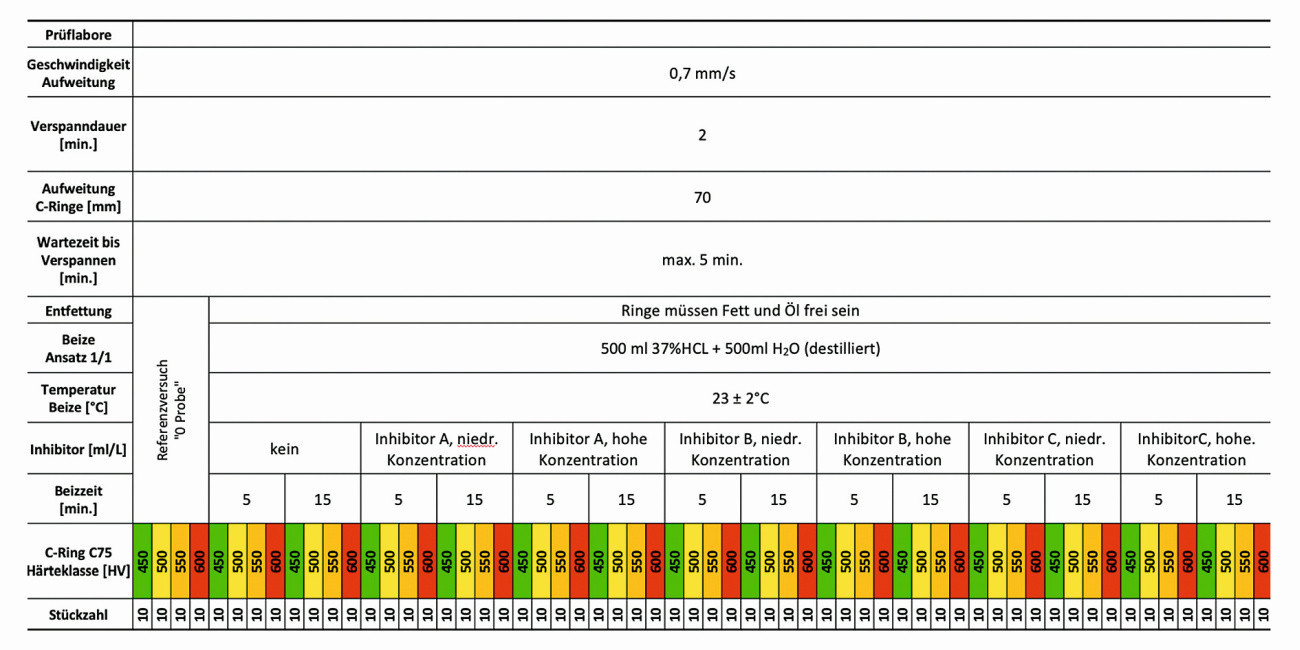 Table 2: Overview of benchmark sample preparation and test conditions for the instrumentalized C-ring tension test (measurement method: constant strain rate)
Table 2: Overview of benchmark sample preparation and test conditions for the instrumentalized C-ring tension test (measurement method: constant strain rate)
Permeation measurement
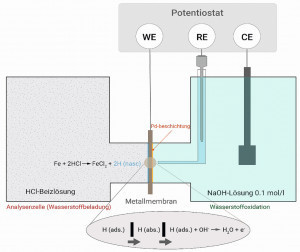 Fig. 6: Basic setup of the electrochemical double cell according to Devanathan and Stachurski A measurement setup according to Devanathan and Stachurski based on DIN 17081 was used for the permeation measurement. This consists of a double cell separated by a membrane (e.g. metal foil DC 04/0.1 mm). The metal membrane is provided with a palladium coating on one side on the hydrogen oxidation side. The membrane, electrodes and potentiostat are arranged and electrically connected as shown in Figure 6. The cell on the palladium side (hydrogen oxidation side) is filled with 0.1 molar sodium hydroxide solution and polarized to hydrogen oxidation potential. Immediately after filling with 0.1 molar NaOH solution, a current jump is measured. Only when a constant quiescent current < 1 μA is reached is the analysis side filled with the medium to be tested (pickling solution in this case). After the atomic hydrogen has diffused through the membrane, an increase in the permeation current can be measured as a function of the membrane thickness and the analysis time that has elapsed. The permeation current results from the leakage of the atomic hydrogen on the palladium-coated side of the membrane and its electrochemical oxidation. This reaction is recorded as a permeation current and the current measured over time provides information about the atomic hydrogen formation on the analysis side of the measuring cell. The amount of charge that has flowed is calculated from the time the hydrogen passes through for 15 minutes (immediately after filling the test solution).
Fig. 6: Basic setup of the electrochemical double cell according to Devanathan and Stachurski A measurement setup according to Devanathan and Stachurski based on DIN 17081 was used for the permeation measurement. This consists of a double cell separated by a membrane (e.g. metal foil DC 04/0.1 mm). The metal membrane is provided with a palladium coating on one side on the hydrogen oxidation side. The membrane, electrodes and potentiostat are arranged and electrically connected as shown in Figure 6. The cell on the palladium side (hydrogen oxidation side) is filled with 0.1 molar sodium hydroxide solution and polarized to hydrogen oxidation potential. Immediately after filling with 0.1 molar NaOH solution, a current jump is measured. Only when a constant quiescent current < 1 μA is reached is the analysis side filled with the medium to be tested (pickling solution in this case). After the atomic hydrogen has diffused through the membrane, an increase in the permeation current can be measured as a function of the membrane thickness and the analysis time that has elapsed. The permeation current results from the leakage of the atomic hydrogen on the palladium-coated side of the membrane and its electrochemical oxidation. This reaction is recorded as a permeation current and the current measured over time provides information about the atomic hydrogen formation on the analysis side of the measuring cell. The amount of charge that has flowed is calculated from the time the hydrogen passes through for 15 minutes (immediately after filling the test solution).
Thermal desorption spectroscopy
A Bruker measurement setup with an IR07 infrared oven and connected mass spectrometer was used for thermal desorption spectroscopy.
The samples were heated in the IR07 infrared oven at a heating rate of approx. 1 K/s. Preliminary tests have shown that at holding times of 5 min in the temperature range of (200 ± 10) °C, the first hydrogen peak can be observed with a low trap energy.
The second hydrogen peak can be observed in the course of the further continuous heating phase starting at approx. 300 °C, with subsequent continuous heating up to 650 °C. Higher temperature ranges > 700-900 °C, on the other hand, showed no significant additional hydrogen effusion. The relationship between thermal sample load and hydrogen effusion is shown schematically in Figure 7.
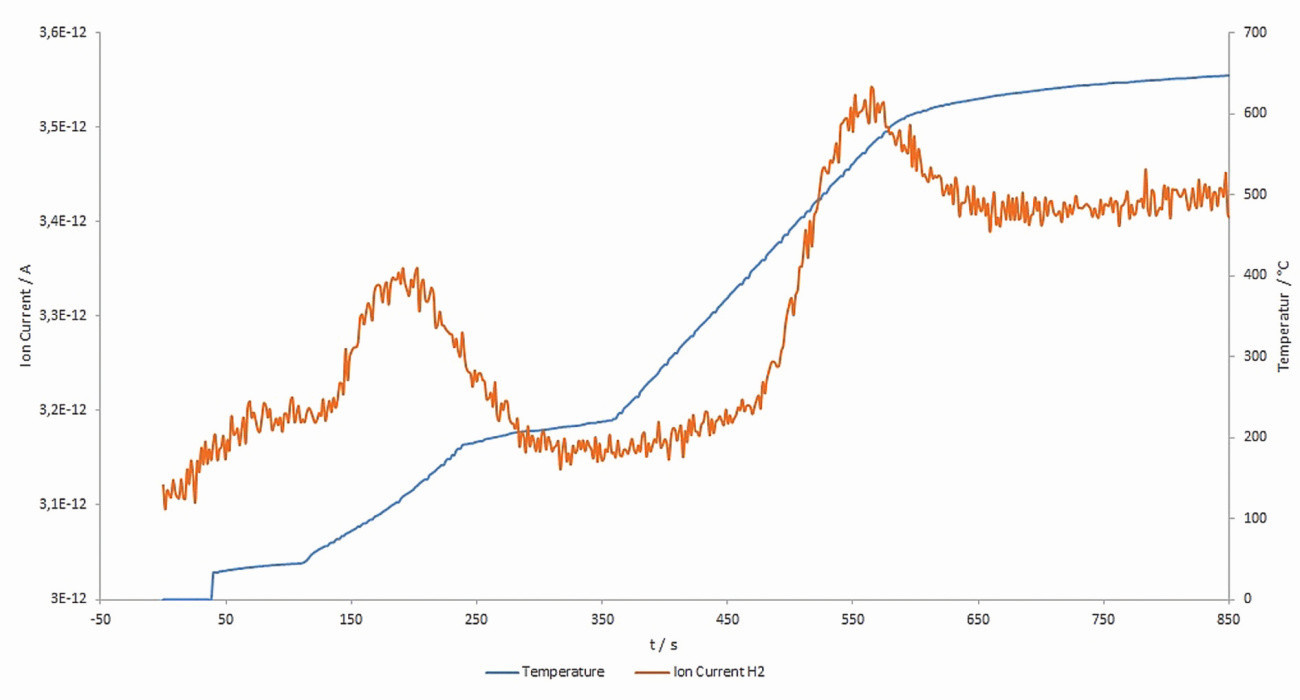 Fig. 7: Schematic relationship between the thermal sample load and hydrogen effusion
Fig. 7: Schematic relationship between the thermal sample load and hydrogen effusion
Results of the C-ring stress test using the constant strain rate measurement method
The results of the stress test on material C75 provided an almost identical picture of the failure rates across all material hardness classes and the pickling inhibitors used, including the specified pickling times (see Fig. 8). The test specimen failure in the non-inhibited initial state of the pickling baths is clearly recognizable across all hardness classes. Only in hardness class 450HV5 was the failure rate of the C-ring test specimens less than 100 % after 5 minutes of pickling in a non-inhibited pickling bath.
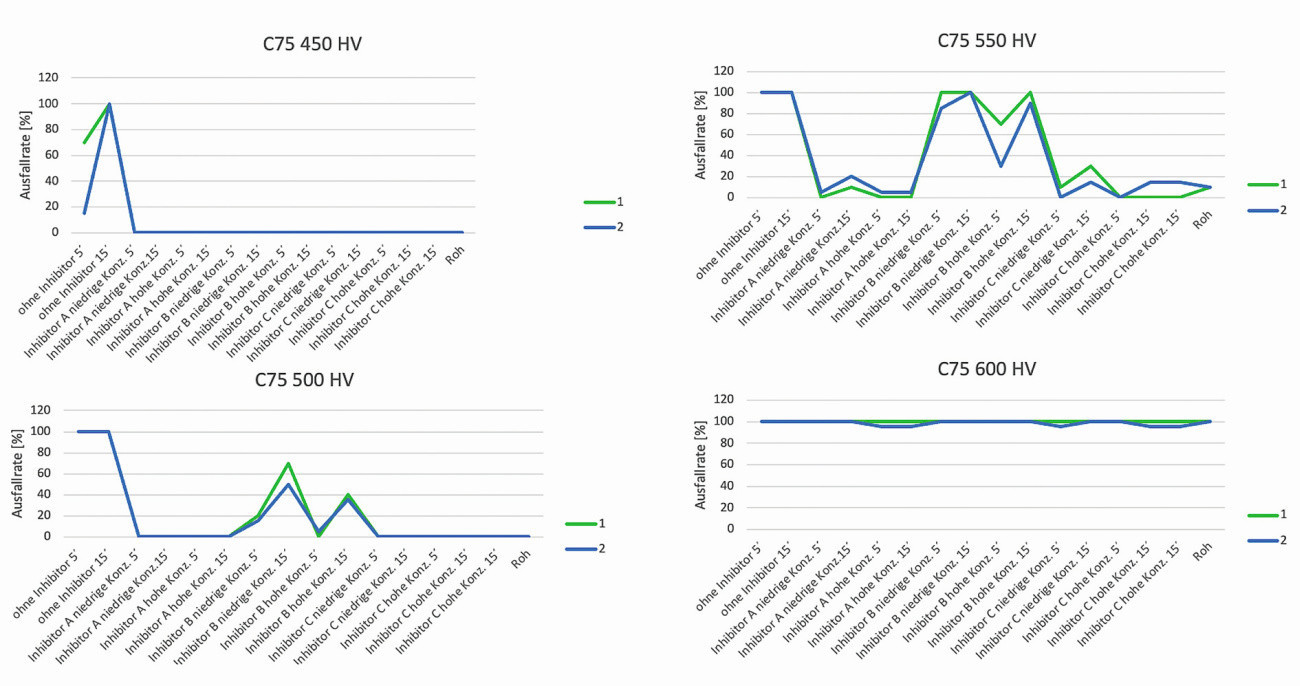 Fig. 8: Illustration of the C-ring test specimen failure rate as a function of the material hardness, the pickling inhibitors used and pickling times
Fig. 8: Illustration of the C-ring test specimen failure rate as a function of the material hardness, the pickling inhibitors used and pickling times
All other tests showed a 100% test specimen failure rate. With the use of pickling inhibitors, a significant reduction in test specimen failure rates can be measured. In strength class 500HV5, test specimen fractures were observed after pickling with inhibitor B both after 5 minutes and after 15 minutes. The remaining tested pickling batches in strength class 500HV5 behaved inconspicuously. The failure rates in the respective test sample batches increase with increasing material hardness. The differentiated effect of the pickling inhibitors is consistent across all pickling tests in strength classes 500HV5 and 550HV5. It is only from strength class 600HV5 onwards that there is an almost 100% failure of the test specimens in all test sample groups. A differentiated assessment of the effect of the inhibitors used is no longer possible solely on the basis of the failure rate at a material hardness of 600HV5. The slight differences in the failure rates can probably be explained by the differences in the acid concentration of the pickling baths (acid concentration laboratory 1: 220 g/L vs. laboratory 2: 205 g/L).
Results of the thermal desorption spectroscopy
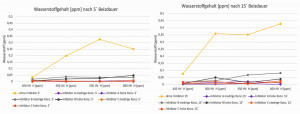 Fig. 9: Diffusible H content (peak 1) after pickling the C-ring test specimens according to the test program The diffusible hydrogen content was determined as part of the same test program as the stress test of the C-ring test specimens.
Fig. 9: Diffusible H content (peak 1) after pickling the C-ring test specimens according to the test program The diffusible hydrogen content was determined as part of the same test program as the stress test of the C-ring test specimens.
Figure 9 shows the diffusible hydrogen content of the first evaluated peak of the desorption spectroscopy. The higher hydrogen loading of the C-ring test specimens after pickling in a non-inhibited pickling solution compared to hydrogen loading after pickling in an inhibited pickling solution is clearly recognizable. In contrast, only slight differences in the hydrogen contents with low trap energy were found in the samples that were pickled with inhibition after the evaluation of the first hydrogen peak.
No differences could be observed in the hydrogen contents of the test specimens after evaluation of the second peak.
Results of thermal desorption spectroscopy (1st H peak) vs. failure rate after stress testing using the CSR measurement method
If a correlation is established between the failure rate and the hydrogen content with low trap energy (effusion temperature < 200 °C), it was found that the failure rate of the C-ring test specimens also increases with increasing H content, regardless of the pickling time. The samples, which only had low H contents after pickling, only showed test specimen failures due to breakage with an offset at higher hardness classes. In the 550HV5 hardness class, for example, no or only very low failure rates can be measured for inhibitors A and C. A direct correlation was found between the failure of the C-ring test specimens and their hydrogen loading (Fig. 10).
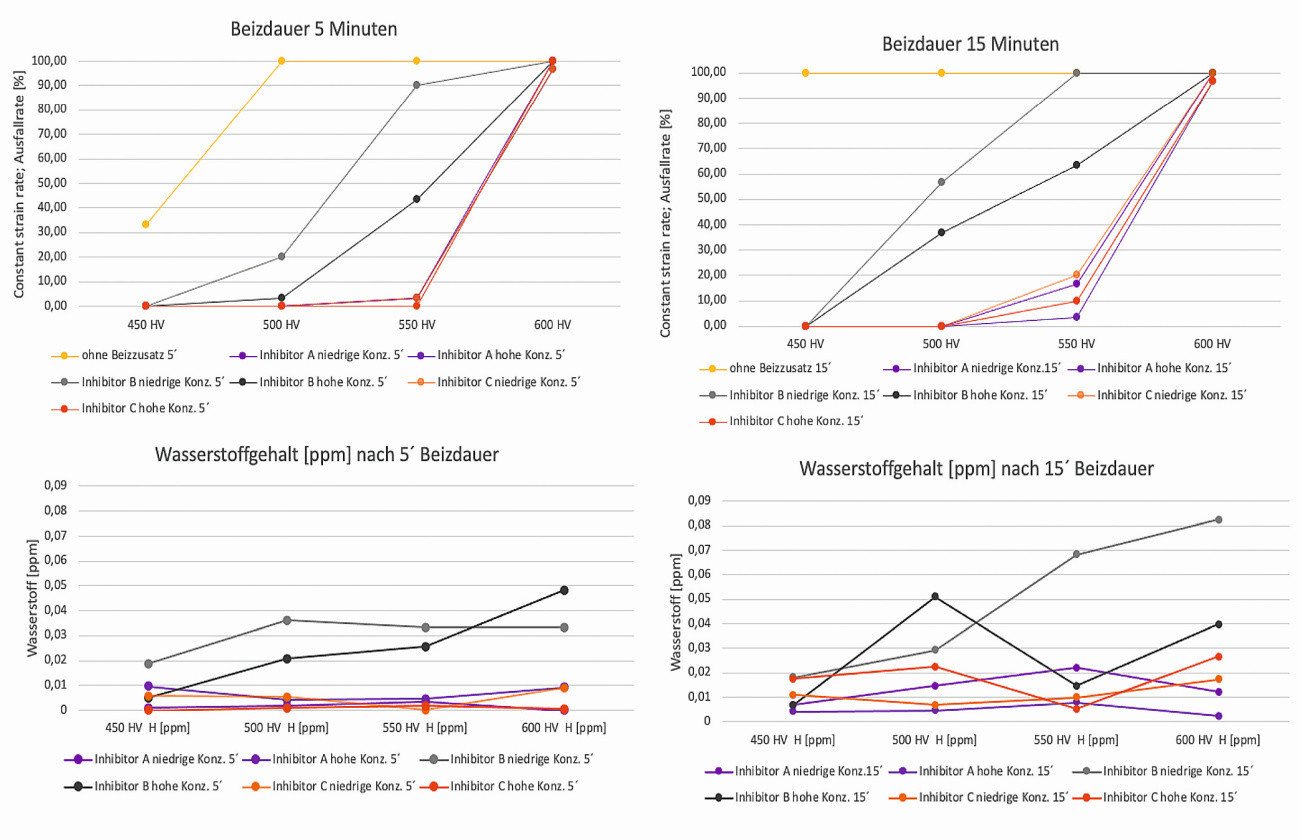 Fig. 10: Correlation between the diffusible hydrogen content of the C-ring test specimens (1st measurement peak) and their failure rate after pickling with different pickling inhibitors and varying pickling times
Fig. 10: Correlation between the diffusible hydrogen content of the C-ring test specimens (1st measurement peak) and their failure rate after pickling with different pickling inhibitors and varying pickling times
Results of thermal desorption spectroscopy (1st H peak) vs. results from permeation tests
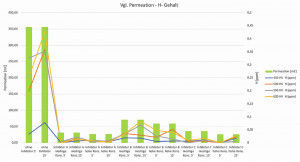 Fig. 11: Correlation between the charge quantity from the permeation tests and the diffusible H content When comparing the flowed charge quantity from the permeation tests with the diffusible hydrogen content of the C-ring test specimens (effusion temperature < 200 °C), good agreement can be seen across all test groups. As expected, the highest charge quantities and hydrogen contents are measured in the non-inhibited test groups. This correlation also continues with the inhibited samples. The highest levels of diffusible hydrogen and the largest amounts of charge were measured in the inhibitor B test group after 5 and 15 minutes of pickling time and at different preparation concentrations. In contrast, test groups A and C showed reduced levels of diffusible hydrogen and the amount of charge flowed was uniformly at a low level of < 40 mC (see Fig. 11).
Fig. 11: Correlation between the charge quantity from the permeation tests and the diffusible H content When comparing the flowed charge quantity from the permeation tests with the diffusible hydrogen content of the C-ring test specimens (effusion temperature < 200 °C), good agreement can be seen across all test groups. As expected, the highest charge quantities and hydrogen contents are measured in the non-inhibited test groups. This correlation also continues with the inhibited samples. The highest levels of diffusible hydrogen and the largest amounts of charge were measured in the inhibitor B test group after 5 and 15 minutes of pickling time and at different preparation concentrations. In contrast, test groups A and C showed reduced levels of diffusible hydrogen and the amount of charge flowed was uniformly at a low level of < 40 mC (see Fig. 11).
Results of the stress test using the CSR measurement method vs. test results from permeation tests
The comparison between the measured charge quantity and the determined failure rates is somewhat more differentiated. The test specimens of hardness classes 500HV5 and 550HV5 show a good correlation between the amount of charge flowed from the permeation tests and the failure rate. Despite a flowed permeation current, no C-rings failed due to breakage in the inhibited test groups of hardness class 450HV5. In the event that this test result can be reproduced, it would be possible to define a critical threshold value for the amount of charge exchanged, above which test specimen failure is only to be expected. For strength class 600HV5, the failure rate for all test groups was between 96 and 100 %. Thus, in this hardness class, no correlation can be derived between the effect of the tested inhibitors and the amount of charge flowing from the permeation tests and the measured failure rates. Here, the failure rates are dominated solely by the high material sensitivity of the test specimens (Fig. 12).
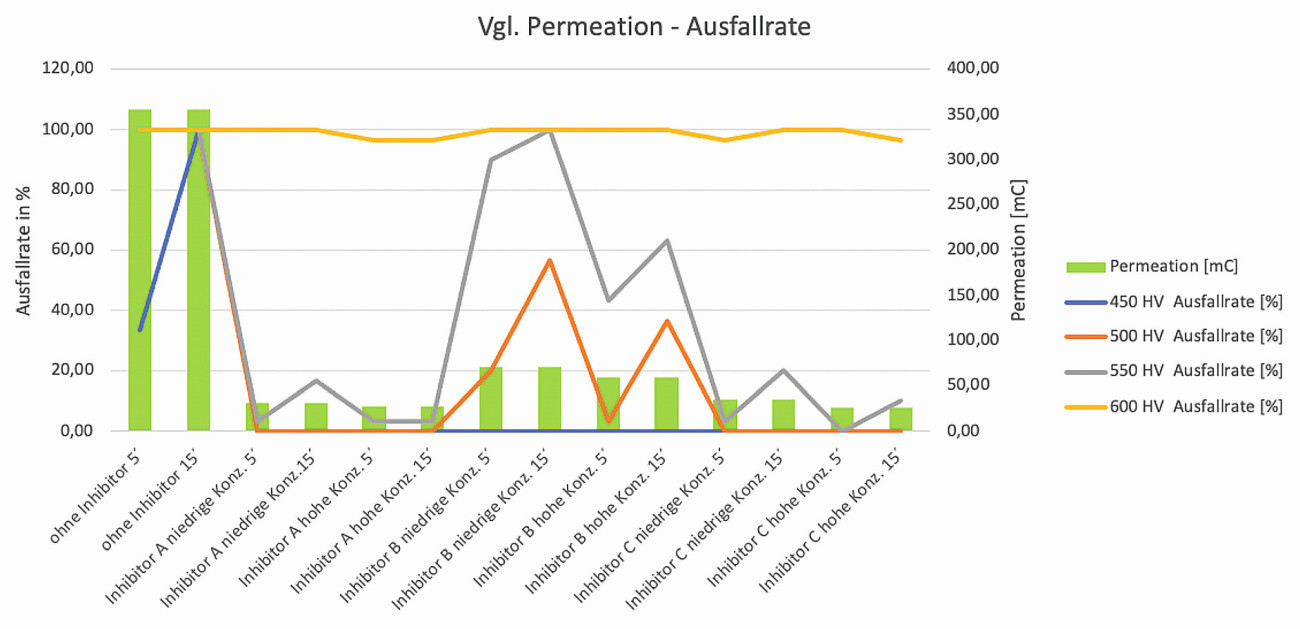 Fig. 12: Relationship between the amount of charge from the permeation tests and the failure rate after stress testing using the constant strain rate
Fig. 12: Relationship between the amount of charge from the permeation tests and the failure rate after stress testing using the constant strain rate
Summary and outlook
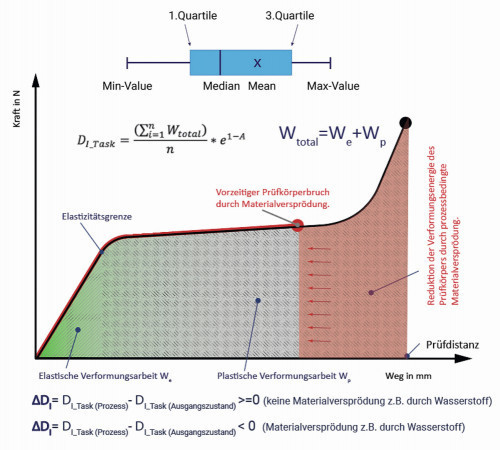 Fig. 13: Illustration of the formation of the deformation index of a test batch derived from the individual force-displacement diagrams of the C-ring test specimens after a mechanical stress test using the constant strain rate measurement method The objective formulated at the beginning of this article to validate a test method that is capable of detecting possible material embrittlement due to production-related hydrogen was successful. The stress test on C-rings made of the material C75 in the strength classes 450HV5, 500HV5, 550HV5 and 600HV5, the permeation measurements and the thermal desorption spectroscopy using a mass spectrometer provided a "cross-check" of the test methods with test results that did not contradict each other. For example, good agreement and reproducibility of the measurement results were found in the distortion tests using the constant strain rate measurement method. Minor deviations in the measurement results can be explained by varying test conditions for the individual benchmark participants. This agreement was also evident when comparing the results from the measurements using thermal desorption spectroscopy (hydrogen absorption test specimen) with the results of the distortion test. The failure rate increased significantly with increasing hydrogen content in the 1st peak at approx. 200 °C. To avoid autocatalytic effects, a limitation of the temperature profile to max. (350 ± 10) °C is suggested for further measurements. In the individual holding stages of 200 and 350 °C, a holding time of 5 to 10 minutes should be aimed for. The permeation measurements also provided good agreement in comparison with the failure rate of the C-ring test specimens and the hydrogen content of the test specimens.
Fig. 13: Illustration of the formation of the deformation index of a test batch derived from the individual force-displacement diagrams of the C-ring test specimens after a mechanical stress test using the constant strain rate measurement method The objective formulated at the beginning of this article to validate a test method that is capable of detecting possible material embrittlement due to production-related hydrogen was successful. The stress test on C-rings made of the material C75 in the strength classes 450HV5, 500HV5, 550HV5 and 600HV5, the permeation measurements and the thermal desorption spectroscopy using a mass spectrometer provided a "cross-check" of the test methods with test results that did not contradict each other. For example, good agreement and reproducibility of the measurement results were found in the distortion tests using the constant strain rate measurement method. Minor deviations in the measurement results can be explained by varying test conditions for the individual benchmark participants. This agreement was also evident when comparing the results from the measurements using thermal desorption spectroscopy (hydrogen absorption test specimen) with the results of the distortion test. The failure rate increased significantly with increasing hydrogen content in the 1st peak at approx. 200 °C. To avoid autocatalytic effects, a limitation of the temperature profile to max. (350 ± 10) °C is suggested for further measurements. In the individual holding stages of 200 and 350 °C, a holding time of 5 to 10 minutes should be aimed for. The permeation measurements also provided good agreement in comparison with the failure rate of the C-ring test specimens and the hydrogen content of the test specimens.
Due to the limited resolution, e.g. in the comparison between the failure rate and the flowed charge quantities, the determination of the deformation energy of the C-ring test specimens is recommended for future stress tests. The deformation energy and the failure rate could be used to demonstrate the embrittling effect of the hydrogen with statistical certainty, taking into account the process parameters and the process chemistry. For example, the intensity of material embrittlement caused by hydrogen could be derived from the reference to a non-embrittled initial state in the same hardness class. The procedure and the basic ideas of the test evaluation are shown in Figure 13. A numerically calculated deformation index can be used to describe the distribution of the deformation energy of the test lot. Both the deformation energy of the test lot, derived from the recorded force/displacement curves, and the failure rate (e1-A) are statistically included in the deformation index. If the test result is set in relation to the untreated initial state of the test batch, it is possible to determine the embrittling effect of the hydrogen in the production process. The characteristic value to be formed would then provide the user with the possibility of process control or the development of effective process chemistry, e.g. pickling inhibitors. In further studies, additional test specimens (e.g. notched C-ring test specimens), test methods (constant load, step load test), pickling additives and practical working parameters (e.g. influence of foreign metals) are to be investigated comparatively.
* Peierls stress is the shear stress required to move a dislocation through a crystal at a given average speed.
Literature
[1] D. Kuron: Wasserstoff und Korrosion, Verlag Irene Kuron Bonn (2000), 7-50
[2] I.M. Robertson et al.: Hydrogen Embrittlement Understood, Metall. Mater. Trans. A, 46(2015)6, 2323-2341
[3] M. Nagumo: Fundamentels of Hydrogen Embrittlement, Springer-Verlag (2016)