4.2.3 Cover layer formation on galvanized steel
In the case of zinc, the protective layer of corrosion products adhering to the surface, which is formed from the reaction of the dissolved zinc ions with the substances from the environment, is referred to as the top layer. The protective capacity is largely dependent on the composition and structure of the coating and the formation conditions. Ideally, the corrosion rate of the zinc falls to values of a few micrometres per year, which is why galvanized steel can achieve a service life of many decades in practice (e.g. in steel construction). In the build-up phase of the top layer, alternating humid conditions are favorable, in which the surface initially corrodes in the presence of water, but then dries again and allows theCO2 from the air access to the corrosion products. These then react to form chemically stable and dense protective layers, usually based on zinc carbonates. Conversely, certain air pollutants and permanently humid conditions have an unfavorable effect on the corrosion rate, as more unstable compounds are formed (zinc compounds with sulfates and chlorides).
Tracking the formation of the coating layer under different climatic conditions or characterizing the current condition of a component or a galvanized steel structure has not been easy to implement until now. Due to the very low corrosion rates, this requires lengthy test programs and complex examinations of the samples. From a practical and scientific point of view, however, very interesting questions arise for which corrosion diagnostics with electrochemical methods using gel-like electrolytes can be used very well. The advantage here also lies in the simple implementation of the electrochemical measurements and the minimally invasive procedure, which means that the condition of the coating does not change significantly during the analysis. This can be illustrated by comparing liquid electrolytes and gel electrolytes of the same chemical composition. Figure 11 shows the course of polarization resistances as a function of the electrolyte used on a galvanized steel sample with an atmospherically formed coating.
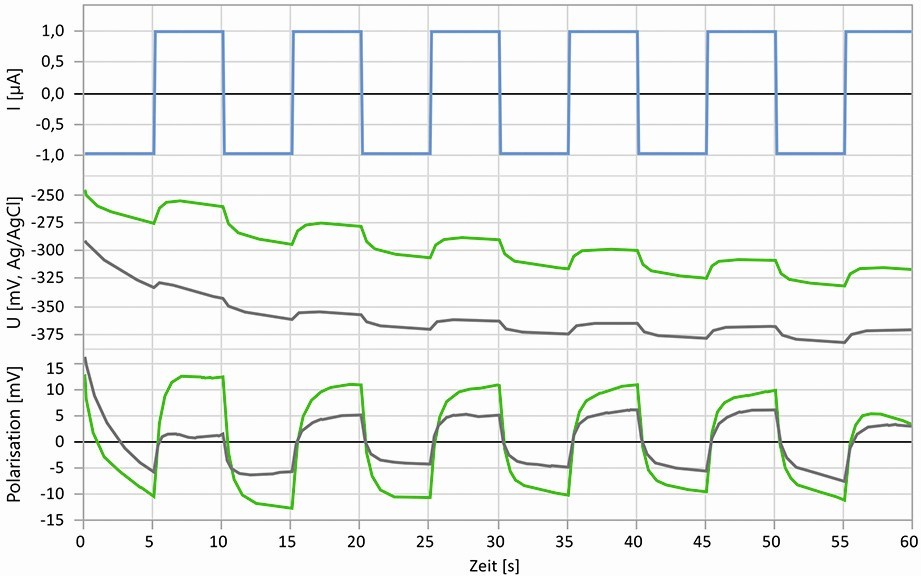 Fig. 10: Minimally invasive galvanostatic pulse measurements over 60 seconds on untreated (gray) steel and steel aged in a VCI atmosphere (green), top: Excitation with pulse current, center: potential curves, bottom: Polarization curves
Fig. 10: Minimally invasive galvanostatic pulse measurements over 60 seconds on untreated (gray) steel and steel aged in a VCI atmosphere (green), top: Excitation with pulse current, center: potential curves, bottom: Polarization curves
The polarization resistances were determined using the galvanostatic pulse method shown in Figure 10, which has only a very slight effect on the surface. After contact with the liquid electrolytes, the polarization resistance drops from around 200 kΩcm2 to 50 kΩcm2 within a few minutes, whereas with the gel electrolytes it remains constantly high for much longer, thus enabling the electrochemical characterization of this state over a longer period of time. The reason for this lies in the property of the gel electrolyte to form a thin electrolyte film on the surface, whereby the corrosion products dissolve much more slowly and their removal from the surface is inhibited by the gel network. This condition is closer to the conditions that occur on a metal surface in a humid atmosphere. Here too, in most cases there is a thin electrolyte film on the surface, which suppresses the removal of corrosion products.
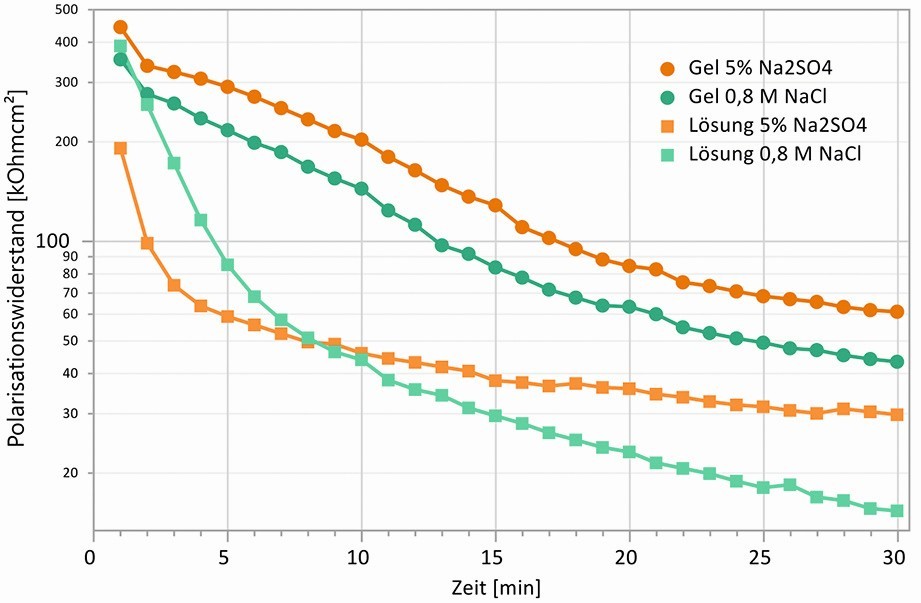 Fig. 11: Progression of polarization resistances over 30 min in contact with gel and liquid electrolytes, galvanized steel with atmospherically (C2 city atmosphere) formed top layer
Fig. 11: Progression of polarization resistances over 30 min in contact with gel and liquid electrolytes, galvanized steel with atmospherically (C2 city atmosphere) formed top layer
Numerous investigations have already been carried out in this way in recent years. In [23], for example, the build-up and stabilization phase of zinc samples under various atmospheric conditions is described using measurements with gel electrolytes. Figure 12 shows an important result of these investigations.
It is impressive that under ideal, alternating wet-dry conditions, the surface layer can form within the first 2-3 weeks and then already enters a stabilization phase in which the corrosion current densities are very low. In contrast, under dry conditions (33 % relative humidity) it remains unfinished and under humid conditions (96 % relative humidity) it also remains constant, but significantly less resistant. The polarization resistances for a stable top layer phase under the C2 atmosphere are well over 100 kΩcm2 (corresponds to significantly less than 0.08 µA/cm2), whereas much lower polarization resistances are determined under the constant climates (0.4 µA/cm2 corresponds to approx. 26 kΩcm2). The use of gel electrolytes to determine surface resistances on zinc coatings is currently being investigated at the BAM Federal Institute for Materials Research and Testing (Berlin) as part of the BMWi's WIPANO research initiative (knowledge transfer through patents and standards) [29] in order to prepare a draft standard for a test procedure.
![Abb. 12: Verläufe der momentanen Korrosionsstromdichten, bestimmt durch Versuche mit gel-artigen Elektrolyten, von unterschiedlich ausgelagerten Zink-Proben, Babutzka et al. [23] Abb. 12: Verläufe der momentanen Korrosionsstromdichten, bestimmt durch Versuche mit gel-artigen Elektrolyten, von unterschiedlich ausgelagerten Zink-Proben, Babutzka et al. [23]](/images/stories/Abo-2022-02/gt-2022-02-0074.jpg) Fig. 12: Curves of the instantaneous corrosion current densities, determined by tests with gel-like electrolytes, of differently aged zinc samples, Babutzka et al [23].
Fig. 12: Curves of the instantaneous corrosion current densities, determined by tests with gel-like electrolytes, of differently aged zinc samples, Babutzka et al [23].
![Abb. 13: Veränderung von Polarisationswiderständen unterschiedlicher Verzinkungen auf Stahl in einem Kurzzeitkorrosionstest der Automobilindustrie, Daten aus [30] Abb. 13: Veränderung von Polarisationswiderständen unterschiedlicher Verzinkungen auf Stahl in einem Kurzzeitkorrosionstest der Automobilindustrie, Daten aus [30]](/images/stories/Abo-2022-02/thumbnails/thumb_gt-2022-02-0075.jpg) Fig. 13: Change in polarization resistances of different zinc coatings on steel in a short-term corrosion test in the automotive industry, data from [30] With regard to the behaviour of galvanized steel under atmospheric conditions, the question often arises as to whether this behaviour can be simulated or even accelerated by tests in artificial, usually more severe climates. The methodology can also be used very well for this question. Although continuous monitoring over the entire course of the test is not possible, it is relatively easy to carry out measurements on the previously removed samples after certain test intervals and thus document the current surface condition (protective capacity of the surface layer) very well. In a study by Killik, this approach was used to examine various galvanized coatings on steel during aging in a typical short-term corrosion test in the automotive industry [30] and compared with field components. Figure 13 shows the polarization resistances of various galvanized coatings over the course of the test. The measurements were carried out in the first week after certain individual cycles of the test and in the long-term range after 1, 2, 4 and 8 weeks. The individual galvanizations are at different levels, but without a pronounced tendency to increase the surface layer resistance. The passivations are very noticeable in the course of the test by increasing the resistances and partially delaying the drop to low values. The normal hot-dip galvanizing corrodes the most in this test (lowest resistances), but also has a higher coating thickness and shows red rust and a drop in polarization resistance at the end of the test period. The electroplated Zn-Fe and Zn-Ni coatings have higher resistances, but the corrosion is still so strong (and the coating thickness is proportionally lower) that it also leads to red rust and thus to a sharp drop in resistance during the test. For all galvanizing variants tested, no coating formation can be detected in this short-term corrosion test, as observed under atmospheric conditions. This is mainly due to the more stringent test conditions (high temperatures, high humidification times, high salt exposure).
Fig. 13: Change in polarization resistances of different zinc coatings on steel in a short-term corrosion test in the automotive industry, data from [30] With regard to the behaviour of galvanized steel under atmospheric conditions, the question often arises as to whether this behaviour can be simulated or even accelerated by tests in artificial, usually more severe climates. The methodology can also be used very well for this question. Although continuous monitoring over the entire course of the test is not possible, it is relatively easy to carry out measurements on the previously removed samples after certain test intervals and thus document the current surface condition (protective capacity of the surface layer) very well. In a study by Killik, this approach was used to examine various galvanized coatings on steel during aging in a typical short-term corrosion test in the automotive industry [30] and compared with field components. Figure 13 shows the polarization resistances of various galvanized coatings over the course of the test. The measurements were carried out in the first week after certain individual cycles of the test and in the long-term range after 1, 2, 4 and 8 weeks. The individual galvanizations are at different levels, but without a pronounced tendency to increase the surface layer resistance. The passivations are very noticeable in the course of the test by increasing the resistances and partially delaying the drop to low values. The normal hot-dip galvanizing corrodes the most in this test (lowest resistances), but also has a higher coating thickness and shows red rust and a drop in polarization resistance at the end of the test period. The electroplated Zn-Fe and Zn-Ni coatings have higher resistances, but the corrosion is still so strong (and the coating thickness is proportionally lower) that it also leads to red rust and thus to a sharp drop in resistance during the test. For all galvanizing variants tested, no coating formation can be detected in this short-term corrosion test, as observed under atmospheric conditions. This is mainly due to the more stringent test conditions (high temperatures, high humidification times, high salt exposure).
4.2.4 Estimating the service life of galvanized steel in outdoor exposure
As has been shown, the methodology and the characteristic values obtained from the tests can be applied and used in a variety of ways. Of scientific interest are studies on the formation of the surface layers under variation of various parameters, such as the exposure conditions or the areas of application, but also the type of zinc coatings. However, many users are also interested in whether these parameters can be used to draw conclusions about the future service life of a galvanized coating in practical use. This question is of course justified and, in principle, it is possible based on the characteristic values determined. This is because polarization resistances and corrosion current densities are characteristic values [31] from which an instantaneous corrosion rate can be calculated by applying Faraday's law and the material characteristics [32]. The question when interpreting a value calculated in this way is whether it represents the future "normal state" or whether the system will continue to change. To estimate the corrosion rate or service life, further assumptions are therefore necessary, namely
- that the formation of the covering layer is complete (stable phase, see Fig. 12) and
- that the media conditions no longer change to such an extent that a renewed degradation and reconstruction of the surface layer occurs.
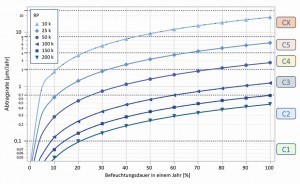 Fig. 14: Relationship between the predicted removal rate for zinc or galvanized steel, the average wetting duration and the determined polarization resistance (RP) For immobile objects (installations in the construction industry), the media conditions vary over a year depending on the season and location, but are relatively constant and easy to determine. To simplify matters, the average wetting time over a year can be assumed (corrosion in the dried state is negligible) and the future corrosion rate and a remaining service life for a zinc coating can be calculated. If this approach is followed, values are obtained that are plausible and lie within the range of the normative removal values for zinc in certain exposure classes, see DIN EN ISO 9223 [33]. Figure 14 illustrates the relationship between the predicted removal rate for zinc, the average wetting duration and the determined polarization resistance. The dashed lines indicate the limit ranges C1 to CX of the removal rates after one year for zinc.
Fig. 14: Relationship between the predicted removal rate for zinc or galvanized steel, the average wetting duration and the determined polarization resistance (RP) For immobile objects (installations in the construction industry), the media conditions vary over a year depending on the season and location, but are relatively constant and easy to determine. To simplify matters, the average wetting time over a year can be assumed (corrosion in the dried state is negligible) and the future corrosion rate and a remaining service life for a zinc coating can be calculated. If this approach is followed, values are obtained that are plausible and lie within the range of the normative removal values for zinc in certain exposure classes, see DIN EN ISO 9223 [33]. Figure 14 illustrates the relationship between the predicted removal rate for zinc, the average wetting duration and the determined polarization resistance. The dashed lines indicate the limit ranges C1 to CX of the removal rates after one year for zinc.
Two practical examples are used to illustrate the relationship. A sample of galvanized steel was exposed to the weather for one year under urban conditions. An average polarization resistance of approx. 150 kΩcm2 was determined on the sample (comparable to Fig. 11, measurement with gel electrolyte). The corrosion current density is calculated from the polarization resistance and from this, taking into account the density and valence of the zinc ion, the amount of zinc that has gone into solution. This is given as mass loss per year andm2 or, assuming uniform metal dissolution, as the removal rate in µm/year. As the surface is only moistened for a certain period of the year, the duration of moistening (τ) is also required, as corrosion only occurs in the presence of water on the surface. According to DIN EN ISO 9223 [33], times with a temperature > 0 °C and a relative humidity > 80 % are defined as "humidified". The average humidification period for the location was determined by the author using weather data from the German Weather Service (DWD) [34]. The calculation with 150 kΩcm2 and an average wetting duration of 34 % results in an average erosion rate of approx. 0.24 µm/year. This value is exactly within the range of exposure class C2, which specifies values of 0.1 to 0.7 µm/year for zinc in the first year of exposure. Since the top layer of the sample has already stabilized after one year, such a low value is plausible. Another example concerns a maritime site (Helgoland Island). Here, too, samples were stored for one year and the surface layer resistance was determined by means of electrochemical measurements with a gel electrolyte. From an average polarization resistance of 50 kΩcm2 and an average humidification period of 63 %, an average future removal rate of approx. 1.74 µm/year is calculated. This value is also plausible, taking into account stationary conditions and exposure class C3 (0.7 to 2.1 µm/year), as is common today in certain maritime areas of Northern Europe. Under these conditions, a zinc coating of 100 µm thickness would have a service life of more than 50 years, assuming constant conditions in the future.
Of course, there are always new special features to be found in each individual case, such as locally changing microclimates, material and design differences, leaching of zinc by precipitation, abrasion and much more. This is due to the fact that corrosion is a system property with many influencing variables that interact with each other. In the case of topcoats, however, the influencing factors of material and environmental conditions combine and result in the coating bearing the history of corrosive stress like a kind of fingerprint. Therefore, the surface layer resistance is a reliable characteristic value from which useful conclusions can be drawn if other conditions are known, such as the estimation of the removal rate and, consequently, the service life of a zinc coating. With regard to the calculation model presented, one can share George Box's view that models are all wrong, but some are useful. In the author's opinion, the methodology presented here and the findings that can be derived from it go further than all previous tests on the atmospheric corrosion of galvanized steel. This is because it is precisely the special features mentioned above that can be recorded and evaluated much more easily in terms of their effects using the metrological approach described, even under practical conditions.
5 Summary
In recent years, gel-type electrolytes have attracted increasing attention and application in the field of corrosion investigations and testing. Their use for corrosion diagnostics opens up many new application possibilities. Electrochemical sensor technology and handling can be significantly simplified, which increases acceptance for practical use. In addition, the metrological characterization of a series of surface engineering measures in corrosion protection, for which there were previously only unsatisfactory solutions, is now possible. Applications such as the Korropad and variants thereof are already established in some industries or are on the way to becoming so. Many scientific questions can also be dealt with more effectively and lead to new findings more quickly.
The fact that agar-based gel electrolytes are ideally suited for corrosion diagnostics is due to their unique properties. Even with a low polymer content, stable gels can be produced in which the liquid electrolyte is immobilized by the gel network with pore sizes in the nanometre range. A thin electrolyte film forms on the surface or between the surface and the measuring electrodes due to syneresis, which enables electrochemical measurements. The gel network also means that the mobility of particles of a certain size is restricted. This increases the detectability of local reactions when the ionic species are complexed and fixed at the site of their formation via color indications. Furthermore, the thin electrolyte film and the inhibited mass transport in the gel mean that corrosion reactions are inhibited and can be analyzed more easily using electrochemical methods. This makes it possible to detect and quantify protective systems with low layer weights, such as the presence of VCI as a temporary corrosion protection measure.
The use of gel electrolytes to determine coating resistances on zinc coatings is another promising application that should lead to a draft standard in the future. The aim is to standardize the handling of the method and make it more comparable. The further processing of the electrochemical characteristic values makes it possible to estimate future removal rates of zinc coatings in outdoor weathering. The calculated values already reflect the real behavior surprisingly well. The sensory effort involved is relatively low and, thanks to advances in the field of mobile electrochemical measurement technology, relatively simple measuring devices are conceivable in the future.
Acknowledgments:
The author would like to thank Ms. M.Sc. Christine Becker (OvGU Magdeburg) for carrying out the measurements on the electrolyte film, Mr. M.Sc. Ludwig Gropler (OvGU Magdeburg) for the 3D design and printing of sensors, iLF Magdeburg GmbH for carrying out the rheological measurements and BAM Berlin (FB 7.6) for the chemical analysis of the agar raw materials. We would also like to thank CORPAC Deutschland GmbH & Co KG and Mubea Fahrwerksfedern GmbH for their excellent and productive cooperation. Special thanks also go to Mr. M.Sc. Sebastian Hütter (OvGU) for programming the image analysis.
Funding reference: Funded by the German Research Foundation (DFG) - project number 330472124.
Literature
[1] Laque, F.L.; May, T.P.; Uhlig, H.H.: Corrosion in Action, International Nickel Company Canada, 1955
[2] Isaacs, H.S.; Adzic, G.; Jeffcoate, C.S.: Visualizing Corrosion, Corrosion 56 (2000) 10, 971-978, https://doi.org/10.5006/1.3294386
[3] DIN EN ISO 10309:2016-08: Metallic coatings - Test method for the determination of porosity - Ferroxyl test (ISO 10309:1994), German version EN ISO 10309:2016
[4] Petersen, P.; Emnéus, H.: The Ferroxyle Test as a General Test of the Corrosiveness of Surgical Appliances Made from Stainless Steel or Co-Based Alloys of Stellite- Type, Mainly Vitallium and Neutrilium, Acta Orthopaedica Scandinavica, 29, 1-4, 1959, 331-340, https://doi.org/10.3109/17453675908988808
[5] Lehmann, J.; Burkert, A.; Müller, T.; Bohlmann, T.; Burkert, A.: Final report on the IGF project 17136 N/1 Detection of corrosion-sensitive surfaces of stainless steels by the processors, available on researchgate.net
[6] Patent specification DE 10 2010 037 775 B4: Condition for the detection of corrosion-sensitive metal surfaces and method for the detection of corrosion-sensitive metal surfaces, patent granted on 8.5.2014
[7] Rosemann, P.; Kauss, N.; Heyn, A.: KorroPad-Prüfung - Anwen[1] dungen aus Industrie und Forschung, 3-Länder-Korrosionstagung - Korrosion ist kein Zufall - Neue Messmethoden, Analytik und Simulation, May 2019, Frankfurt a. Main, available on researchgate.net
[8] Rosemann, P.; Müller, T.; Babutzka, M.; Heyn, A.: Influence of microstructure and surface treatment on the corrosion resistance of martensitic stainless steels 1.4116, 1.4034, and 1.4021, Materials and Corrosion, 66, 2015, 45-53, https://doi.org/10.1002/maco.201307276
[9] Reinemann, S.; Babutzka, M.; Rosemann, P.; Lehmann, J.; Burkert, A.: Influence of grinding parameters on the corrosion behavior of austenitic stainless steel, Materials and Corrosion, 70, 2019, 1776-1787, https://doi.org/10.1002/maco.201910874
[10] Kauss, N.; Heyn, A.; Halle, T.; Rosemann, P.: Detection of sensitization on aged lean duplex stainless steel with different electrochemical methods, Electrochimica Acta 317 (2019) 17-24, https://doi.org/10.1016/j.electacta.2019.05.081
[11] Newton, C.J.; Sykes, J.M.: A galvanostatic pulse technique for investigation of steel corrosion in concrete, Corrosion Science, 28, No. 11, 1988, 1051-1074, https://doi.org/10.1016/0010-938X(88)90101-1
[12] Spark, A.J.; Cole, I.; Law, D.; Ward, L.: The effect of peptide based nutrients on the corrosion of carbon steel in an agar based system, Corrosion Science 110, 2016, 174-181, https://doi.org/10.1021/acs.est.7b00437
[13] Spark, A. J.; Cole, I.; Law, D.; Marney, D. and Ward, L.: Investigation of agar as a soil analogue for corrosion studies. Materials and Corrosion 67 (2016), pp 7-12, https://doi.org/10.1002/maco.201508312
[14] Spark, A.J.; Law, D.W.; Ward, L.P.; Cole, I.S.; Best, A.S.: Effect of Pseudomonas fluorescens on Buried Steel Pipeline Corrosion, Environmental Science & Technology, 51 (15), 2017, 8501-8509, https://doi.org/10.1021/acs.est.7b00437
[15] Vanbrabant, J.; van de Velde, N.: Industrial application of an electrochemical corrosion test using a gel matrix as simulation for atmospheric and solid media, Proceedings: European General Galvanizers Association Intergalva Berlin, Vol. 19, June 7th, 2000, 29/1 to 29/13
[16] Shao, X.M.; Feldman, J.L.: Micro-agar salt bridge in patch-clamp electrode holder stabilizes electrode potentials, Journal of Neuroscience Methods 159, 2007, 108-115, https://doi.org/10.1016/j.jneumeth.2006.07.001
[17] Monrrabal, G.; Guzmán, S.; Hamilton, I.E.; Bautista, A.F.; Velasco, F.: Design of gel electrolytes for electrochemical studies on metal surfaces with complex geometry, Electrochimica Acta, Volume 220, December 2016, 20-28, http://dx.doi.org/10.1016/j.electacta.2016.10.081
[18] Monrrabal, G.; Ramírez-Barat, B.; Bautista, A.; Velasco, F.; Cano, E.: Non-Destructive Electrochemical Testing for Stainless-Steel Components with Complex Geometry Using Innovative Gel Electrolytes. Metals,8, 2018, 500, https://doi.org/10.3390/met8070500
[19] Monrrabal, G.; Huet, F.; Bautista, A.: Electrochemical noise measurements on stainless steel using a gelled electrolyte, Corrosion Science, Volume 148, March 2019, Pages 48-56, https://doi.org/10.1016/j.corsci.2018.12.004
[20] Monrrabal, G.; Bautista, A.; Valesco F.: Use of Innovative Gel Electrolytes for Electrochemical Corrosion Measurements on Carbon and Galvanized Steel Surfaces, CORROSION, Vol. 75, No. 12, December 2019, 1502-1512, https://doi.org/10.5006/3309
[21] Cano, E.; Crespo, A.; Lafuente, D.; Ramirez Barat, B.: A novel gel polymer electrolyte cell for in-situ application of corrosion electrochemical techniques, Electrochemistry Communications, 41 (2014), 16-19, https://doi.org/10.1016/j.elecom.2014.01.016
[22] Di Turo, F.; De Vito, C.; Coletti, F.; Mazzei, F.; Antiochia, R.; Favero, G.: A multi-analytical approach for the validation of a jellified electrolyte: Application to the study of ancient bronze patina, Microchemical Journal, Vol. 134, 2017, 154-163, https://doi.org/10.1016/j.microc.2017.05.015
[23] Babutzka, M.; Burkert, A.; Heyn, A.: Korrosionsuntersuchungen mit gelartigen Elektrolyten zur Beschreibung der Korrosionsschutzwirkung von Zinküberzügen, 16th Summer Course on Materials and Joining: Magdeburg, September 8-9, 2017, 119-128, http://dx.doi.org/10.25673/5002
[24] Babutzka, M.; Heyn, A.: Dynamic tafel factor adaption for the evaluation of instantaneous corrosion rates on zinc by using gel-type electrolytes, IOP Conf. Ser.: Mater. Sci. Eng. 181, 2017, 012021, https://doi.org/10.1088/1757-899X/181/1/012021
[25] Labille, J.; Fatin-Rouge, N.; Buffle, J.: Local and Average Diffusion of Nanosolutes in Agarose Gel: The Effect of the Gel/Solution Interface Structure, Langmuir, 23, 2007, 2083-2090, https://doi.org/10.1021/la0611155
[26] Vaucher, S.; Li, M.; Mann, S.: Synthesis of Prussian Blue Nanoparticles and Nanocrystal Superlattices in Reverse Microemulsions, Angew. Chem. Int. ed, 39, 1793-1796 http://www.doi.org/10.1002/(SICI)1521-3773(20000515)39:10<1793::AID-ANIE1793>3.0.CO;2-Y
[27] Ogston, A.G.; Preston, B.N.; Wells, J.D.: On the transport of compact particles through solutions of chain polymers, Proc. R. Soc. London, A. 1973333, 1973, 297-316, https://doi.org/10.1098/rspa.1973.0064
[28] Somma, M.; Querci M.: The Analysis of Food Samples for the Presence of Genetically Modified Organisms, Session 5: Agarose Gel Electrophoresis, 62, https://doi.org/10.2760/5277
[29] Draft standard for a test method for the determination of surface layer resistances on zinc coatings using gel-like electrolytes - GELELEK, research project in the BMWi WIPANO research initiative, Project Management Jülich, Berlin
[30] Killik, A.: Influencing factors on the corrosion of differently galvanized steel test specimens in short-term corrosion tests and in the field, Dissertation, Otto von Guericke University Magdeburg, 2016
[31] J.R. Scully: Polarization Resistance Method for Determination of Instantaneous Corrosion Rates, Corrosion Vol. 56, No. 2, 2000, 199-218, NACE International, https://doi.org/10.5006/1.3280536
[32] ASTM G3-14: Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing, Reapproved 2019
[33] DIN EN ISO 9223:2012: Corrosion of metals and alloys - Corrosivity of atmospheres - Classification, determination and estimation
[34] Heyn A.: Bewertung der Korrosivität von Atmosphären anhand von Wetterdaten, 16th Summer Course Materials and Joining: Magdeburg, September 8 and 9, 2017, 129-138, http://dx.doi.org/10.25673/5002


