In many cases, coating errors lead to high reject rates. This is particularly annoying when you consider that the coating process usually takes place towards the end of a production chain. If a product becomes unusable or unsaleable at this point, this results in high costs, which increase the later they are discovered. Accordingly, great importance should be attached to quality and process reliability, starting with the selection of the coating system. The following cases of damage, which DFO Service GmbH was commissioned to investigate, are examples of some of the causes.
Fluctuations in gloss level
Modern paint production has high standards in terms of process reliability and quality control, but a certain susceptibility to errors is in the nature of things: paints are mixtures of a variety of raw materials, all of which are subject to their own production process-related fluctuations in terms of homogeneity and consistent quality. In addition, paint production is often divided into many individual steps, each of which introduces its own standard error into the process. Developing a coating formulation with sufficiently precise production instructions and suitably large process windows in order to achieve a consistent and less error-prone coating quality is the art of coating development, which has little to do with exact science. In fact, most coating defects are rarely due to poor paint quality. Nevertheless, this happens time and again and certain manufacturing steps and paint properties stand out as the cause.
In the case of steel components from an automotive supplier, there were areas of varying gloss levels on the individual components after painting. The variations in gloss level affected all components and showed no recognizable pattern. However, the defect could be narrowed down to a specific batch of paint. Although this suggested a connection between the occurrence of the defect and the paint used, the paint manufacturer rejected the possibility of a paint problem. The reason given was that the defect pattern could not be reproduced in laboratory painting tests and the formulation of the batch complained about corresponded to the original formulation without any significant deviations. These arguments are often used when it comes to the question of a possibly defective batch of paint. However, experience has shown that the quantity ratios of the individual paint components are of very little significance in relation to such issues. A comparison of production recipes and protocols, on which production instructions and parameters are typically noted, is more interesting. Anomalies in stirring or dispersing times, actual quantities of raw materials added, unusual solvent additions etc. can, for example, be indications of errors in production or fluctuating raw material quality. A certain amount of leeway must always be taken into account, as even the slightest variations in the process can lead to a behavior of the coating batch that needs to be adjusted. One example of this would be an increased solvent requirement due to a higher-viscosity raw material batch of a film former - in this case, solvent components from later production stages may be preferred in order to guarantee the further processability of the coating formulation. A second example concerns the tinting of a paint batch if a certain color shade needs to be achieved. Due to the difficulty of tinting paints, complex color shades often involve several individual tinting steps, which rarely result in the same quantities being added. Experienced experts are required to distinguish between potentially serious production errors and normal and necessary production fluctuations, as the transition is fluid.
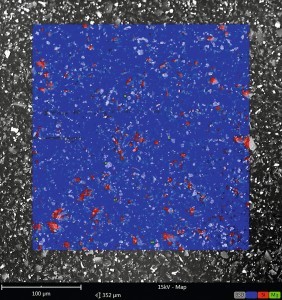
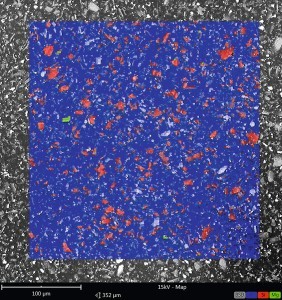
Fig. 1: EDX mapping of the shinier surface (left) and the matt surface (right) in top view
Adjustment tests by painting test panels in a laboratory are also only of limited value, at least if the corresponding defect pattern could not be recreated. The painting conditions are generally not comparable with those in the actual painting process, or only to a very limited extent. This applies, among other things, to the painting parameters, drying conditions, substrates used (test panels instead of original components), the application technique, etc. Accordingly, an argument based on the results of readjustment tests should only be considered in support of further tests and/or analyses.
Consequently, the defect pattern had to be analytically characterized in the next step, which was carried out in the DFO laboratory.
Energy dispersive X-ray spectroscopy (EDX) was used to compare areas with a higher gloss level with areas with a lower gloss level.
The EDX mappings in false color rendering revealed that silicon was detectable in significantly higher quantities on the surface in the duller area of the coating than in the shinier area (see Fig. 1). The control of the gloss level using fumed silicon dioxide works by structuring the coating surface through the SiO2 particles, which results in a higher roughness and therefore a lower gloss level. In order to achieve a homogeneous gloss level across the entire component, the distribution of these matting agents must therefore also be homogeneous. One possibility of inhomogeneous matting is if the coating is too thin. It can happen that the coating thickness falls below the maximum particle size of the fumed silicon dioxide. In this case, the particles protrude further out of the coating surface, increasing the roughness and further reducing the gloss level. A glance at the material data sheet for the coating showed a target coating thickness of 30 µm. Coating thickness measurements on the affected components showed coating thicknesses of 29-32 µm. These were therefore within the target coating thickness range. The same coating thicknesses were measured on unaffected components that had been coated with a different batch of paint. Conversely, it was now necessary to check whether the grain fineness of the SiO2 particles in the supposedly defective batch of paint was so high that it exceeded the target coating thickness.
A simple grindometer test of the conspicuous paint batch provided the explanation: the grain fineness was just under 30 µm. Paint batches that did not show the defect pattern had grain sizes of less than 20 µm. However, with target coating thicknesses of 30 µm, a grain size of just under 30 µm is clearly too high, as there can always be slight fluctuations in coating thickness. If the SiO2 particles are larger than the coating thickness, they protrude from the coating and produce a matt surface.
The cause of the defect was therefore insufficient dispersion or grinding of the matting agent. The grain fineness is usually checked during the production process and noted in the production log. If the grain fineness is above the corresponding target value, a further grinding pass is usually carried out. In this case, the paint manufacturer did not provide an insight into the corresponding production log.
Loss of adhesion
The possible causes of loss of adhesion are numerous and often not easy to determine. A distinction can be made between two basic manifestations of adhesion problems: Either the coating delaminates almost completely from the substrate or from the underlying coating (adhesive loss of bond strength). Or there is cohesive loss of adhesion, in which the coating breaks and delaminates. In most cases, the following causes are by far the most common:
- An unsuitable coating structure: Adhesion forces are based on chemical interaction between the coating material and the substrate surface. If these are not coordinated, sufficient adhesive strength cannot be achieved.
- Inadequate pre-treatment: Conversion coatings typically have a significantly increased surface area, which improves the adhesive strength of a subsequent coating. Gaps or missing conversion layers therefore lead to a reduced adhesive strength of the coating. Inadequate process control during pre-treatment can lead to chemically inhomogeneous or even brittle conversion layers, for example.
- Insufficient cleanliness of the substrate: Contamination between the substrate and coating (e.g. salts, grease, oils, etc.) prevents the formation of adhesive forces between the coating and substrate.
- Insufficient curing of chemically curing coating systems: Undercrosslinking of chemically curing coating systems (e.g. 2K coatings, powder coatings) results in a reduction in chemical resistance and reduced adhesion strength, among other things. This can be caused, for example, by poor mixing or an incorrect mixing ratio of the paint components of 2K paints.
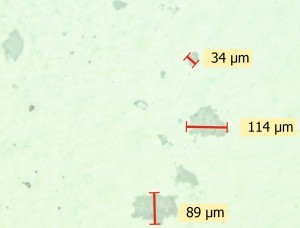 Fig. 2: Light microscopy image of the underside of the top coat with localized deposits However, faulty paints also lead to adhesion problems in individual cases. The following case study was submitted to DFO to clarify the cause.
Fig. 2: Light microscopy image of the underside of the top coat with localized deposits However, faulty paints also lead to adhesion problems in individual cases. The following case study was submitted to DFO to clarify the cause.
In the case of painted plastic components with a two-coat structure, adhesion problems between the primer and the top coat were found over a large area. Since the components were coated in a paint shop that had only recently been put into operation, it was initially assumed that there were problems with the application technology. Various painting tests were carried out with numerous variations in the painting parameters, including the high voltage in the electrostatically assisted application and the curing conditions. However, there was no significant improvement in the adhesive strength of the coating. In order to definitively rule out the paint shop as the cause of the defect, the paint material was applied on the paint shop of a supplier. The defect pattern could be reproduced there.
At the same time, both the topcoat underside and the primer surface were analyzed in the DFO laboratory after delamination. Using light microscopy, it was possible to determine that although the top coat had detached from the primer with virtually no residue, larger fragments of residue were still adhering to the underside in isolated areas (see Fig. 2).
Using IR spectroscopy and energy dispersive X-ray spectroscopy (EDX), no foreign substances could be detected. This led to the conclusion that the interfering substance must be present in very small quantities, below the detection limits of IR spectroscopy and EDX. However, very small amounts of an interfering substance are sufficient to block the formation of adhesive forces between the substrate and the coating in order to cause adhesion problems. For this reason, the samples were additionally analyzed using TOF-SIMS (time-of-flight secondary ion mass spectrometry). This is a method for trace analysis that is suitable for detecting and identifying organic and inorganic atoms and molecules in very small quantities. The result was unequivocal: in the delamination area, concentrations of an organofunctional ammonium compound were detected, which is used, for example, as a rheology additive for paints. In the course of subsequent discussions with the client, it was established that the occurrence of the adhesion problems coincided with an adjustment to the paint system declared by the paint manufacturer.
When adapting paint formulations by adding a new raw material, compatibility with the paint system must always be checked. If paint components can be easily incorporated into a formulation on a laboratory scale, this does not necessarily mean that this will also work without complications on a production scale. For this reason, at least the rudimentary coating properties, such as adhesion, must be re-tested even in the case of minor formulation changes. In addition, special attention must always be paid to abnormalities during the initial production of the adapted coating formulation, which is why production monitoring by the coating developer is generally advisable.
The paint manufacturer was therefore confronted with the analytical results. However, the detected ammonium compound is also used in cosmetics and many other products, so that the paint manufacturer doubted a connection with his paint system and assumed that the substance was introduced into the process during the painting process via the ambient air, with the source "cosmetics".
As the loss of adhesion occurred over a very large area, it was difficult to trace an external contamination via the detour of air contamination with cosmetic residues. In such cases, it must always be assumed that the simplest and most contradiction-free explanation is also the most probable (Occam's razor). Therefore, to detect the ammonium compound in the ambient air, aluminum foil was laid out in the painting area and examined after 24 hours. No ammonium compounds could be detected on the foil surfaces.
The adhesion problems could therefore be clearly attributed to the rheology additive, which was unsuitable for this paint formulation and application.
Powder coating with innovative light protection
 Fig. 3: Sample sheet with adhesion problems after cross-cut and reverse impact test The following case (insurance claim) concerns powder-coated glass bottles that were coated by a job coater for his customer. The bottles were used as packaging for light-sensitive liquids. The powder coating manufacturer had therefore added light protection additives to the slightly tinted clear powder coating to protect the contents of the bottles from light. With this type of bottle, there was a "reproducible" (i.e. every batch produced was affected to a greater or lesser extent) loss of adhesion of the coating over time. Comparable bottle types that had been coated with other powder coatings (without light protection additives) did not show any loss of adhesion.
Fig. 3: Sample sheet with adhesion problems after cross-cut and reverse impact test The following case (insurance claim) concerns powder-coated glass bottles that were coated by a job coater for his customer. The bottles were used as packaging for light-sensitive liquids. The powder coating manufacturer had therefore added light protection additives to the slightly tinted clear powder coating to protect the contents of the bottles from light. With this type of bottle, there was a "reproducible" (i.e. every batch produced was affected to a greater or lesser extent) loss of adhesion of the coating over time. Comparable bottle types that had been coated with other powder coatings (without light protection additives) did not show any loss of adhesion.
The damage pattern could be reproduced on sample sheets after "damp room storage" (240 h at 40 °C and 100 % relative humidity). The damp room storage in the climate chamber was intended to simulate the stress on the bottles at an accelerated rate, as the loss of adhesive strength occurred with a time delay.
Both the cross-cut test and the "reverse impact test" (ball drop test) resulted in a loss of adhesive strength in the sample sheets (see Fig. 3).
Further analytical tests were then carried out. In this case, ToF-SIMS analysis was selected in order to detect even the smallest amounts of possible substances that could interfere with the adhesive strength. Using this analytical method, high quantities of light stabilizers were detected in the area of the loss of adhesion both on the underside of the coating and on the remaining substrate surface. Consultation with the manufacturer of the light stabilizing additives used finally brought the solution to the problem: The light stabilizing additives used are known to migrate very easily through the coating. They also have a softening effect. If these substances are used in excessive quantities, the light stabilizing additives concentrate at the coating/substrate phase boundary, resulting in the present damage pattern. The case was finally clarified by asking the manufacturer of the powder coating about the quantity of additive used. The paint manufacturer had added a significantly higher quantity of the light protection additive as a modification to the base coat system than recommended by the additive manufacturer. The reason for this was the intention to shield the UV radiation with a desired target layer thickness of 40-50 µm so that the contents of the bottle would not be damaged. Unfortunately, the modified coating system was not tested again with regard to its mechanical-technological properties before use.
Strange inclusions in the coating
After a raw material manufacturer called DFO, it was initially unclear whether DFO would be able to find the cause of the discussed damage case "inclusions in the coating". The raw material manufacturer explained that they had supplied the resin to the coating manufacturer for the production of a styrene-modified acrylate powder clear coat. Small specks had subsequently appeared in the coating at the paint processor. The paint processor had examined these inclusions in his laboratory and determined that an increased styrene concentration could be detected in the area of the inclusions using IR spectroscopy. This made it sufficiently clear to the paint manufacturer that the "culprit" had to be the raw material manufacturer. The raw material manufacturer then tried to reproduce the fault pattern with the affected batch, but was unsuccessful. It was therefore agreed that the raw material manufacturer would bear the costs of the damage. At the same time, however, it was agreed that the damage should be investigated by a "third party" in order to clearly and conclusively determine the cause. It was also agreed that neither party would claim any further costs, regardless of the outcome of the investigation. This agreement included the compensation already paid.
The aim of the investigations was to fully clarify the case of damage with the following boundary conditions:
- Identification of possible contamination/foreign substances in the specks
- Clarification of the source of the specks
- Reproduction of the damage pattern
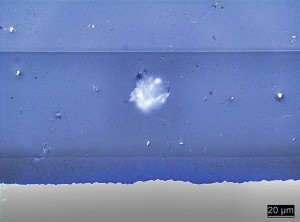 Fig. 4: Cross-section of a defect area with a particle embedded in the coating The DFO naturally approached the clarification of the damage pattern with an open and unbiased mind, even though there had already been extensive investigations into the case. This is the only way to avoid focusing too much on one or a few possible causes of the defect and excluding other potential causes without checking them. This means that all previous investigations were initially called into question. In particular, the preparation of the investigated defects was examined to determine whether the inclusion had actually been investigated. In addition, readjustment tests were carried out by adding polystyrene to increase the styrene content in the powder coating. These did not lead to comparable defect patterns.
Fig. 4: Cross-section of a defect area with a particle embedded in the coating The DFO naturally approached the clarification of the damage pattern with an open and unbiased mind, even though there had already been extensive investigations into the case. This is the only way to avoid focusing too much on one or a few possible causes of the defect and excluding other potential causes without checking them. This means that all previous investigations were initially called into question. In particular, the preparation of the investigated defects was examined to determine whether the inclusion had actually been investigated. In addition, readjustment tests were carried out by adding polystyrene to increase the styrene content in the powder coating. These did not lead to comparable defect patterns.
During the visual and light microscopic assessment of the coating surface by the DFO, it was noticeable that significantly more defects were visible during the visual assessment than with the light microscope. Experience has shown that this indicates that some of the particles are located below the coating surface.
New cross-sections of defects were produced using a grinding and polishing process of samples embedded in casting resin. After preparing the samples, the assumption of particles embedded in the coating was confirmed (see Fig. 4).
In total, both inclusions that were deep in the coating and those that protruded from the coating were found.
The subsequent IR spectroscopic examination was first carried out on particles that protruded from the coating. The results of the IR spectroscopy initially appeared very unusual. Everything indicated that the enclosed particles consisted of a polypeptide. The IR spectra detected best matched gelatine. However, as gelatine is not a typical paint component, there were initially doubts about the correctness of the investigations. Even extensive research did not lead to a direct link between gelatine and powder coating production or any coating components.
In damage analysis, however, "being open to everything" also means examining all possibilities, however improbable and absurd they may seem at first. At this point, it was therefore initially assumed that it was actually gelatine. What this had to do with an increased styrene content, as determined in the initial investigations by the paint processor, was not clear up to this point. The possibility was therefore considered that the particle itself had not been examined in the initial investigations. Rather, the DFO assumed that the particle had fallen out of the defect site during preparation at the paint processor and that the powder coating had been examined at the site where the particle had previously been located. Conversely, this would mean that instead of the particle, possible cleavage products of the clear coat were examined with the particle that was no longer present.
IR spectroscopy mapping was used to verify this theory. In this method, several hundred IR spectra are recorded over the area under investigation and evaluated with regard to local homogeneities and chemical composition.
In this investigation, it was indeed found that the intensities of the absorption bands typical of styrene increase in the transition area between the clear coat and the particles (see Fig. 5).
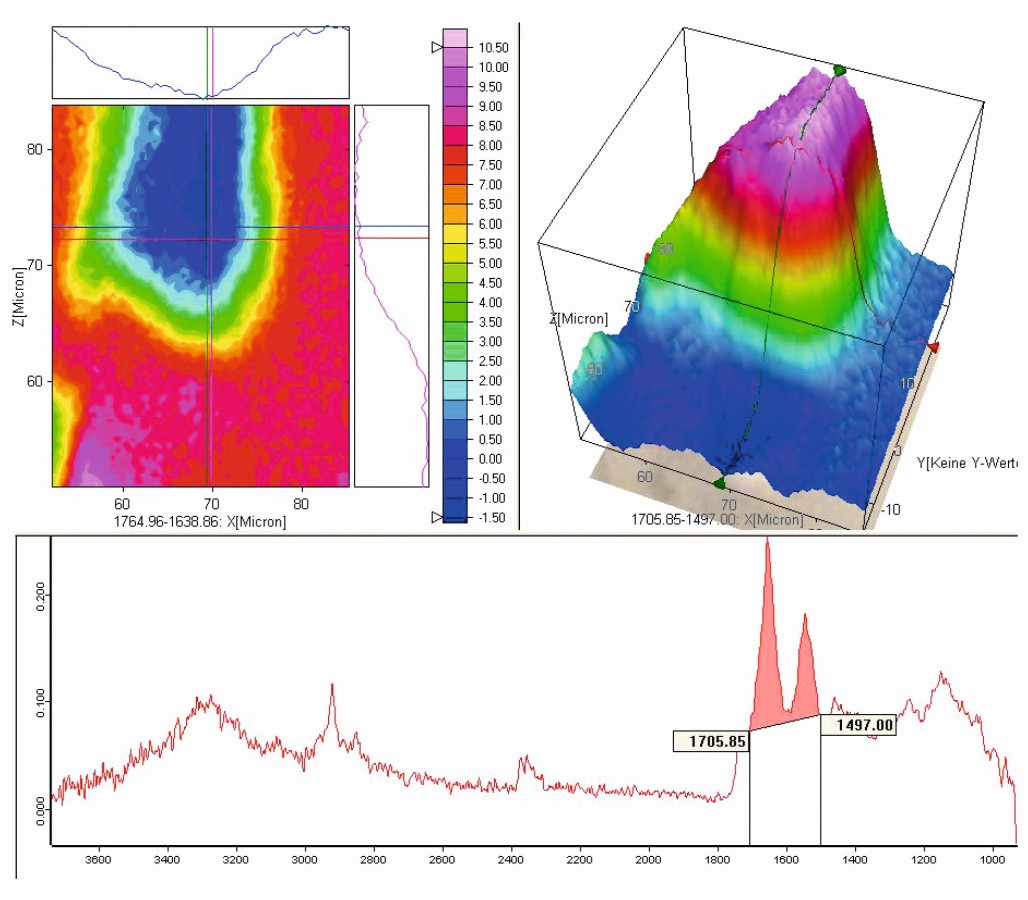 Fig. 5: IR spectroscopy mapping in the defect area in false color representation - distribution and intensity of styrene-typical bands
Fig. 5: IR spectroscopy mapping in the defect area in false color representation - distribution and intensity of styrene-typical bands
This confirmed the assumption that it was not the particle but the remaining powder coating matrix that was examined in the first investigation.
It was now necessary to clarify the contamination path with the gelatine particles that were actually causing the defect. During the discussion with one of the English-speaking raw material manufacturers, potential sources of gelatine had to be quickly defined. Initially, the DFO only thought of gummy bears. However, the English translation was missing at first: "In Germany we call it 'Gummibärchen' ...". The English-speaking conversation partner immediately knew what it was about: "Oh, Haribo". Thus the idea of gummy bears was born, which was then also named in the final report.
During re-adjustment tests with gelatine (not gummy bears), the entry point could also be assigned very precisely. The gelatine was added to the powder coating at various points in the production process. A comparable defect pattern could only be produced when the gelatine was added during the grinding process.
The presentation of the results was surprisingly free of discussion. After the presentation of the results and the unusual source identified, there were no questions from either party involved. Obviously the paint manufacturer had already come up with the same idea himself or was thinking of employees who like to eat jelly babies.