Aluminum facade cladding can be given photocatalytic properties by incorporating titanium dioxide nanoparticles and thus reduce air pollution with nitrogen oxides (NOx). Against this background, the DECHEMA Research Institute, in cooperation with the Research Institute of Precious Metals+Metal Chemistry and the Leibniz University of Hanover, carried out a research project from 2019 to 2021 to develop photocatalytically active aluminum surfaces.
Introduction
The emission of nitrogen oxides (NOx) is currently one of the main problems for air quality in cities. This is the conclusion reached by the German Federal Environment Agency (UBA) in its evaluation of annual average NOx values, according to which in 2019 around 12% of measuring stations in Germany exceeded the European limit values for nitrogen dioxide (NO2) on an annual average [1]. Against the background of a tightening of the limit values from 40 µg/m3 to 10 µg/m3 recommended by the WHO, as many as 88 % of all measuring stations are above this level [2]. Around 60 % of NOx emissions are attributable to the mobility and residential sectors. For this reason, the DECHEMA Research Institute (DFI), in cooperation with the Research Institute Edelmetalle+Metallchemie (fem) and Leibniz Universität Hannover (TCI), carried out a research project from 2019 to 2021 as part of industrial joint research (IGF 20136 N) to develop photocatalytically active aluminum surfaces.
Aluminium is an important construction material in the building industry and is often used - in anodized form - for large-scale cladding of facades and roofs. To reduce nitrogen oxides, photocatalytic reactions can be initiated under the influence of daylight, which convert harmful NOx into nitrate (NO3-). The nitrate formed on the surface is easily soluble in water and is absorbed and transported away by condensation, rainwater or cleaning water on a façade. Each photocatalytically coated aluminum façade could therefore contribute to reducing the concentration of nitrogen oxide in the air and thus improve the quality of life. This principle is illustrated schematically in Figure 1.
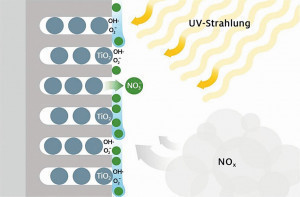 Fig. 1: Operating principle of pollutant degradation on anodized aluminium façades
Fig. 1: Operating principle of pollutant degradation on anodized aluminium façades
This property is already being used in some building materials such as photocatalytically active roof surfaces, façade coatings, coated paving stones and tunnel linings [3].
The possibilities for large-scale photocatalytic coating of aluminum facades in the architectural sector are enormous. The opportunities for a new product class of pollutant-reducing façade concepts combined with the material aluminum are enormous and offer considerable market potential for the aluminum processing industry.
In contrast to iron or low-alloy steels, aluminum is highly resistant to corrosion. The reason for this is the natural oxide layer on the metal surface, which forms in the air and is up to 15 nm thick without additional treatment [4]. As the corrosion-protective properties of the natural passive layer are limited, its thickness is technically increased by the process of "electrolytic oxidation of aluminum (ElOxAl)". The layer is usually produced electrolytically using sulphuric acid, oxalic acid or phosphoric acid processes [5-8]. The resulting mesoporous oxide layer structure is often used to decoratively color aluminum facades by electrolytically incorporating metal salts of tin, nickel or organic or inorganic dyes into the pore structure [9, 10]. The progress made in recent years in the development of colloidal systems makes it possible to use the open-pored structure of anodized aluminium to create new functional properties through electrophoretic integration. The principle is similar to the coloring of anodized layers and is based on the incorporation of nanoparticles into the anodized pores [11-13]. The production of photocatalytically active TiO2 nanoparticles [14, 15] in the size range < 20 nm and their commercial availability on the market [16, 17] are also essential for the implementation.
When a photocatalyst is irradiated with sunlight (such as on a house facade), reactive charge carrier pairs (electrons and holes) are formed, which react with oxygen or water molecules in a first reaction step, whereby highly reactive superoxide (O2-) or hydroxyl (OH-) radicals are formed, which can completely oxidize, i.e. degrade, pollutant molecules during their further reaction [18].
To classify the photocatalytic activity, products are tested according to ISO 22197-1 and the relative photocatalytic efficiency (rPCE) is determined. If an rPCE value of at least two is achieved, the nitrogen oxide degradation is sufficient and the product is beneficial to environmental and health protection due to its air-purifying effectiveness.
The work presented here shows how synthesized titanium dioxide nanoparticles dispersed in suitable solvents can be incorporated into oxalic acid anodization layers by means of electrophoretic deposition. The functionalized layers were then investigated with regard to their photocatalytic activity and corrosion properties.
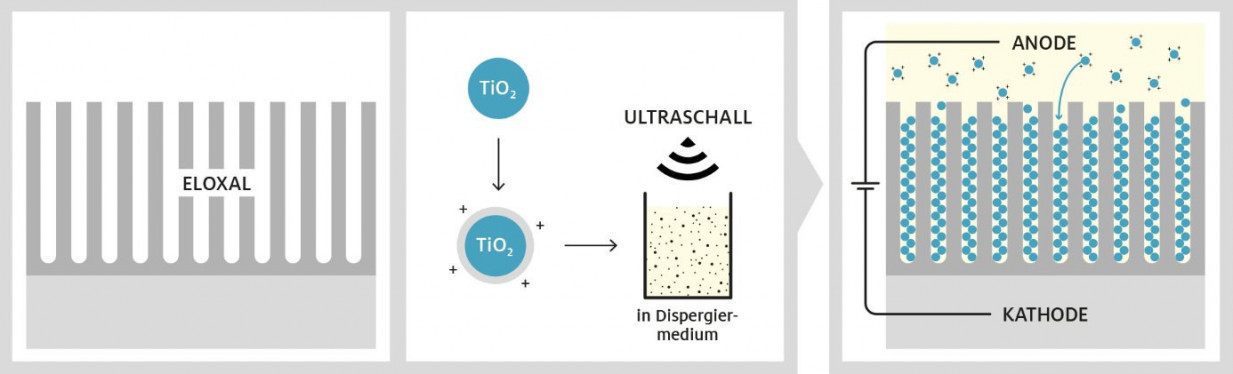 Fig. 2: Work steps for creating the photocatalytically active anodized surface
Fig. 2: Work steps for creating the photocatalytically active anodized surface
Experimental
The procedure for producing photocatalytically active anodized surfaces consists of three basic steps: the targeted structuring of anodized surfaces (1.), the synthesis of photocatalytically active titanium dioxide particles (2.) and their incorporation into the anodized pores (3.)(Fig. 2).
The decisive factor here is the production of the smallest possible, photoactive TiO2 nanoparticles(ideal: particle sizes ≤ 20 nm), with simultaneous variation of the surface charge (zeta potential) in different solvents.
By filling the pores created with nanocrystalline titanium dioxide, the barrier properties of the anodizing can be improved in terms of corrosion protection. On the other hand, the large surface area or porosity of the photocatalyst required for high activity can be maintained and the relatively weakly anchored nanoparticles are also effectively protected against abrasion by the solid "aluminum oxide pillars".
Particle production
The titanium dioxide nanoparticles are prepared by reacting titanium tetrachloride with benzyl alcohol according to a known synthesis procedure and dispersed in water after precipitation [19]. In a typical synthesis, 120.93 g (1.135 mol) of benzyl alcohol was introduced and a mixture of 30.93 g (0.678 mol) of ethanol and 12.36 g (0.066 mol) of titanium tetrachloride was added. The solution was then heated under reflux until a cloudy, yellow solution was formed. The titanium dioxide was then precipitated with diethyl ether (approx. 700-1000 mL). The solid was centrifuged off, washed several times with diethyl ether and then taken up with water. In this way, solutions with a TiO2 content of about 20% by weight were prepared. The hydrodynamic diameter of the nanoparticles in the synthesized solution is approximately 20 nm. The zeta potentials are between 30 and 40 mV at 20 wt%. After dilution with dimethyl sulfoxide (DMSO), homogenization is carried out using the Sonifier W 250 D ultrasonic homogenizer for 3 min at an amplitude of 60 % (pulse: 1 s, pause: 2 s).
Oxalic acid anodization
Depending on the electrolyte and the deposition conditions, anodized layers have a more or less ordered, parallel pore structure and are known to have a high open porosity in the uncompacted state. The pore structure is intended to act as a "container" for the electrochemically deposited titanium dioxide nanoparticles so that the photocatalytic properties of the surface are retained even after abrasive cleaning of the facade. Aluminum sheets made of the alloy AlMg1 (EN AW-5005) were used to produce structured aluminum oxide layers. Prior to anodization, the aluminium substrates were degreased in an alkaline degreasing process at 50 °C for 4 min, pickled in a sodium hydroxide solution (E6 pickling solution) and decapitated in a sulphuric acid process. The pickling process was carried out for 5 min at 50 °C, while the decapitation was carried out at RT for 1 min. Following pre-treatment, anodization was carried out using direct current. A stainless steel sheet (V2A) was connected as the cathode and the aluminium sheet to be anodized as the anode. The surface area of the cathode is 135 cm2 and the surface area of the aluminum sample is 25 cm2. The electrodes were contacted above the bath level. The influence of different anodization conditions is described later in the article.
Electrophoretic deposition
In electrophoretic deposition (EPD), an electric field is applied to the system consisting of nanoparticle suspension and anodized aluminium, causing the nanoparticles to migrate into the pore structure of the anodized aluminium and become embedded there.
The experimental setup for electrophoretic deposition consists of a rectifier, a cold thermostat and an EPD cell. The temperature of the double-walled test cell is controlled with the cold thermostat, while the suspension is circulated with a magnetic stirrer. The electrophoretic intercalation takes place at a distance of 1.5 cm, with the graphite anode (area: 7.5 cm2) and the anodized surface (area: 5 cm2) connected as a cathode being contacted above the bath surface. After electrophoretic immersion, the samples are rinsed in deionized water and then in an ethanol-acetone mixture (50/50 vol.%).
Determination of the photocatalytic activity
The photocatalytic activity of the anodizing layers filled with TiO2 particles is determined by measuring the NOx degradation in accordance with ISO 22197-1.
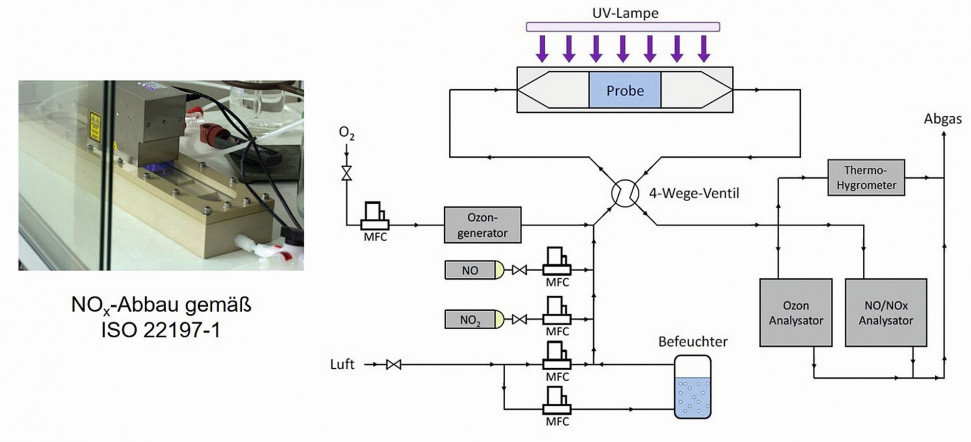 Fig. 3: Schematic structure of the measuring apparatus for investigating the photocatalytic activity of anodizing layers filled with TiO2 particles
Fig. 3: Schematic structure of the measuring apparatus for investigating the photocatalytic activity of anodizing layers filled with TiO2 particles
Figure 3 shows the schematic structure of the measuring apparatus used for this purpose and a photo of the reactor in which the samples are placed. The gas flow during the measurement is 3 l/min at a relative humidity of 50 % and an initial NO concentration of 1 ppm. The irradiation with UV light in the reactor has a power of 1 mW/cm2. The total irradiated reactor area is 50 cm2 and the measurement duration with the UV lamp switched on is 5 hours.
The measurement curves are first used to determine the absolute amount of NO degraded over the course of 5 hours. The so-called rPCE value is then calculated from this. This is the ratio of the absolute amount of substance degraded to the maximum degradable amount of substance in the test gas multiplied by a factor of 75 [20]. The maximum degradable amount of substance corresponds to the amount of substance passed through the reactor within the irradiation time (5 hours). The rPCE value indicates the relative photocatalytic efficiency. Products for photocatalytic air purification that achieve an rPCE value of at least 2 can be designated as "photocatalytically active for air purification in accordance with the FAP voluntary commitment" [21].
Corrosion resistance
The photocatalytically active anodized coatings modified with TiO2 particles are intended for use in façades. Therefore, the corrosion resistance to a commercially available aluminum cleaner (Clever-AS-Technik GmbH) is examined. This has a pH value of 1.8 in a typical dilution of 1:20 specified by the manufacturer. A standard 3-electrode setup in an Avesta cell with vertical sample installation and an Ag/AgCl electrode as reference electrode and a platinum sheet as counter electrode is used for the corrosion tests. The measurements are carried out with a Zahner Zennium potentiostat. At least two measurements are carried out on different sample areas for each sample. The measurement sequence consists of a 60-minute resting potential measurement, followed by an impedance measurement in the range of 10 mHz-100 kHz with an amplitude of 10 mV and a final potentiodynamic polarization in the range of -50 mV to + 400 mV relative to the resting potential (EOC) with a scan rate of 0.5 mV/s.
Results and discussion Generation of meso- and macroporous surfaces
The focus of this publication is on meso- to macroporous surfaces with a pore diameter between 40-60 nm. These surfaces are obtained by oxalic acid anodization at an electrolyte concentration of 57 g/L and a temperature of 20 °C in a one-step process. The anodization conditions are based on several publications [22-26] and were empirically further developed as part of the research project. As accessibility to the pore is restricted during anodization in oxalic acid, the anodized layers were subjected to post-treatment ("pore expansion") in phosphoric acid. This results in the chemical re-dissolution of the aluminum oxide, which increases the diameter of the entire anodized pore. At the same time, the aspect ratio of pore diameter to cell diameter can be varied (see Fig. 4).
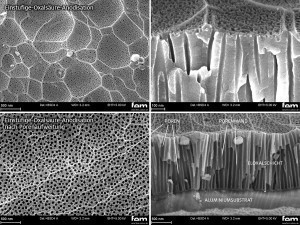 Fig. 4: Effect of pore expansion on an industrial oxalic acid layer in top view (left) and in fracture (right)
Fig. 4: Effect of pore expansion on an industrial oxalic acid layer in top view (left) and in fracture (right)
For the subsequent electrophoretic incorporation of nanoparticles into the meso- and macroporous surfaces, not only the pore diameters were varied, but also the degree of branching of the pores was adjusted. Two methods were used for this purpose: industrial, single-stage anodization with branching and two-stage anodization to produce branching-free pores, in which highly ordered pore structures are formed [22, 27].
The latter variant is realized by pre-structuring the aluminium substrate. This has already been described by Masuda and Fukuda [25, 28], who produced highly ordered meso- and macroporous aluminium surfaces by anodizing aluminium in sulphuric acid, oxalic acid or phosphoric acid electrolytes [22, 27, 29]. Identical process parameters for one-step anodization were used to produce highly structured surfaces. After the first anodization step, the oxide layer is then removed with a chromium phosphoric acid (20 g/L CrO3 and 35 ml/l 85% H3PO4) at 50 °C. The removal time depends on the layer thickness. The detachment time depends on the thickness of the anodized layer and was determined empirically. A structured aluminum substrate remains, which, as described in the literature [30], is due to re-dissolution effects during the pore formation of the anodized layer. This structure is decisive for the highly structured anodized surface without cross-branching produced in the subsequent step. The second anodization was carried out at 0 °C under otherwise identical anodization conditions. The resulting surface is shown in Figure 5.
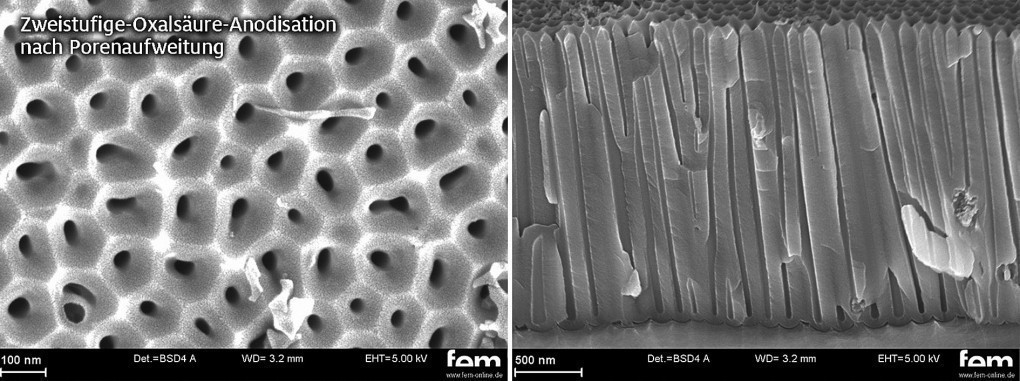 Fig. 5: Top view (left) and fracture (right) of anodized surface after second-stage oxalic acid anodization
Fig. 5: Top view (left) and fracture (right) of anodized surface after second-stage oxalic acid anodization
Electrophoretic intercalation and photocatalytic activity
With the help of parameter tests and statistical test planning, it was possible to achieve good and reproducible intercalation results in oxalic acid layers. Figure 6 shows the pore structure of a crushed EN AW-5005 substrate filled with TiO2 nanoparticles. The particles were deposited in particulate form up to the bottom of the pore. Slight accumulations of TiO2 can be seen on the surface, but these can be avoided by further optimization of the electrophoretic intercalation.
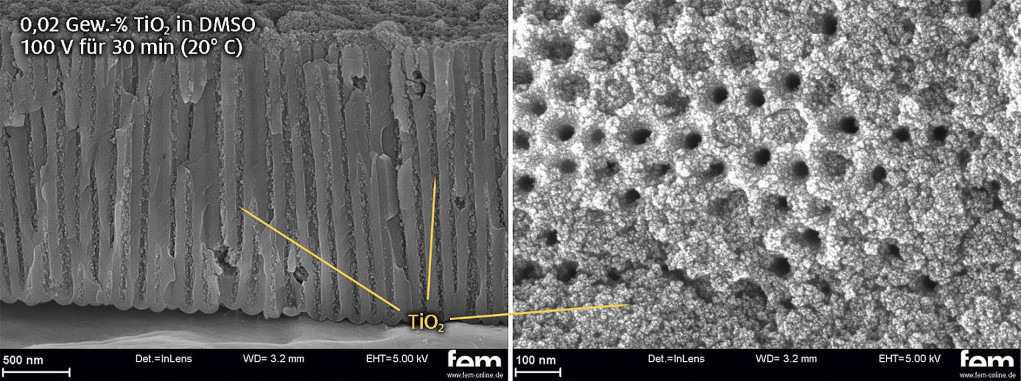 Fig. 6: SEM image of particle intercalation in an oxalic acid layer (one-step anodization) in fracture (left) and top view (right)
Fig. 6: SEM image of particle intercalation in an oxalic acid layer (one-step anodization) in fracture (left) and top view (right)
In the next step, the transferability of the results to EN AW-6060 is checked. Figure 7 shows that with identical process parameters, TiO2 nanoparticles can also be detected within the pore structure of the EN AW-6060 substrate. It is therefore assumed that the transferability of the results is guaranteed if the anodization properties of the substrate material are good. Even if no top layer formation above the anodized layer is visible in the fracture, the whitish, visual appearance of the sample indicates a slight top layer formation. This top layer does not have sufficient abrasion resistance and can be prevented by reducing the storage time, among other things.
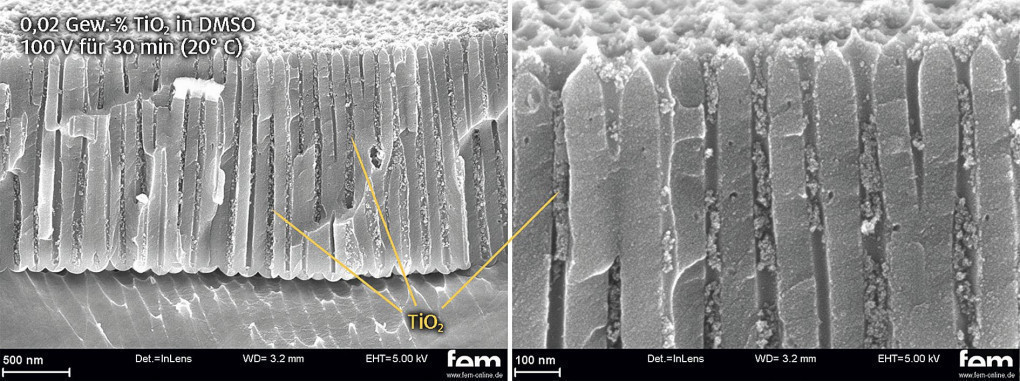 Fig. 7: SEM image of the fracture surface of the EPD results in oxalic acid layers (two-stage anodization) on an EN AW-6060 substrate
Fig. 7: SEM image of the fracture surface of the EPD results in oxalic acid layers (two-stage anodization) on an EN AW-6060 substrate
From the results of the statistical test design, it was possible to determine the significant influencing variables of stress and time as well as the interaction between stress and time. By varying the voltage, it was not only possible to influence the impregnation depth and the deposited mass of TiO2 particles, but also to change the packing density of the particles in the pore. Thus, at low voltages (60 V) it is possible to produce compact particle inclusions and at higher voltages (175 V) particulate inclusions (see Fig. 8).
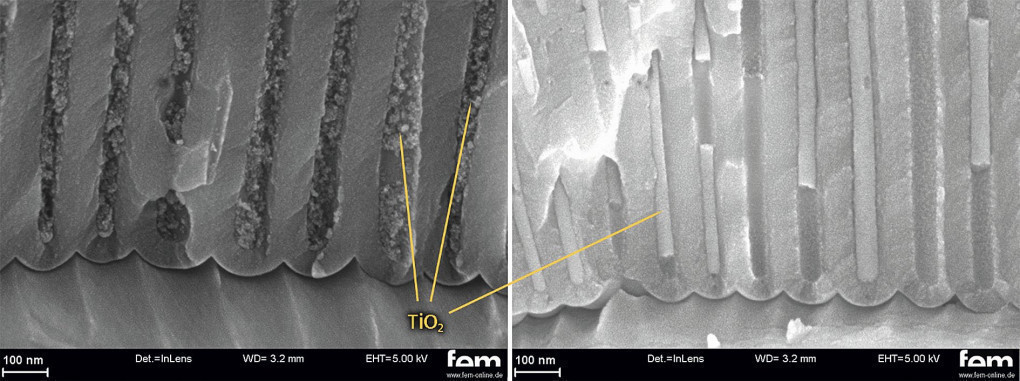 Fig. 8: SEM image for comparative observation of a particulate (left; 0.02 wt.%TiO2, 200V, 15 min) and a compact intercalation (right; 0.2 wt.%TiO2, 100V, 90 min) in an oxalic acid layer (two-stage anodization)
Fig. 8: SEM image for comparative observation of a particulate (left; 0.02 wt.%TiO2, 200V, 15 min) and a compact intercalation (right; 0.2 wt.%TiO2, 100V, 90 min) in an oxalic acid layer (two-stage anodization)
This behavior has already been observed in layer formation using EPD [19, 31, 32] and is attributed by Besra et al [33] to turbulence in the suspension, which occurs when higher voltages are applied and changes the layer formation mechanism.
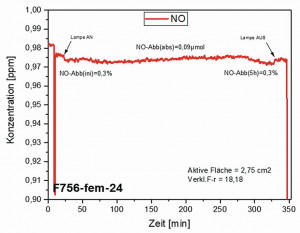 Fig. 9: Nitric oxide degradation curves of particulate intercalation in oxalic acid layers
Fig. 9: Nitric oxide degradation curves of particulate intercalation in oxalic acid layers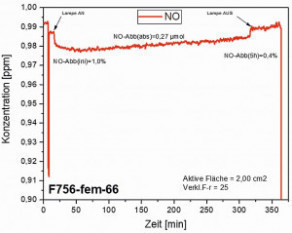 Fig. 9: Nitric oxide degradation curves of compact intercalation in oxalic acid layers
Fig. 9: Nitric oxide degradation curves of compact intercalation in oxalic acid layers
Once the functionalized surfaces had been produced, the photocatalytic activity was determined. Exemplary measurements according to ISO 22197-1 (see chapter on photocatalytic activity), which are recorded to determine the amount of NO degraded, are shown in Figure 9. In the case of particulate intercalation, the absolute NO degradation is 1.68 µmol, which corresponds to an rPCE value of 3.2. The absolute NO degradation in the oxalic acid layer with compact particulate intercalation is 6.78 µmol, which corresponds to an rPCE value of 12.8. Contrary to expectations, compact incorporation has a relative photocatalytic efficiency (rPCE) that is four times higher than with particulate particle incorporation. In accordance with the voluntary commitment of the Association for Applied Photocatalysis to achieve a minimum activity for NO degradation on photocatalytically active surfaces, manufacturers only undertake to advertise a product as photocatalytically active if an rPCE value of at least 2 is achieved. This target value was clearly exceeded in both cases.
Due to the good intercalation results in highly ordered pore structures without cross-branching, the next step was electrophoretic intercalation in industrial pore structures (single-stage anodization). In the initial state, no intercalation of TiO2 is possible due to the small pore opening on the surface of the oxalic acid layer. For this reason, expansion with phosphoric acid is necessary for electrophoretic intercalation.
Figure 10 shows the intercalation results with and without pore expansion. Although it is possible to intercalate TiO2 into the mesoporous pore structure, the intercalation results are significantly worse than for the highly ordered layers with identical process parameters. This is attributed to the cross-branching of industrial coatings, which has a negative influence on the intercalation result.
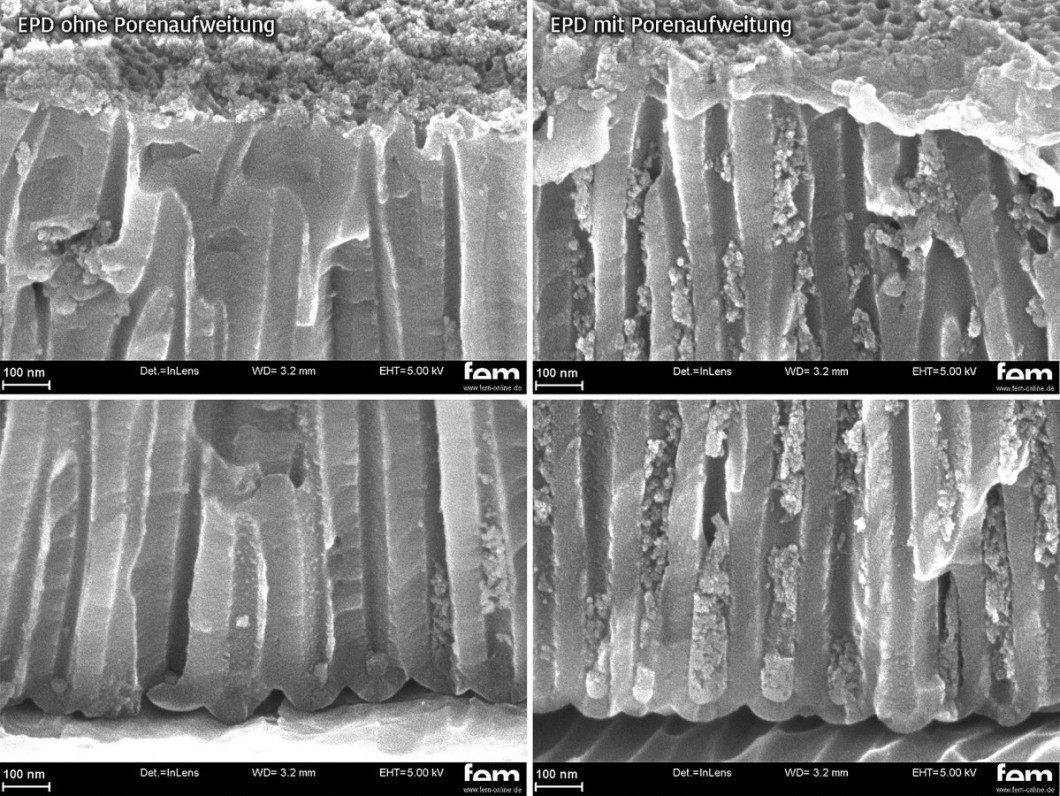 Fig. 10: SEM images of an oxalic acid layer (single-stage anodization) without (left) and with expansion in phosphoric acid (right), EPD parameters: 0.2 wt.%, 20°C, 340 rpm, 100 V, 90 min
Fig. 10: SEM images of an oxalic acid layer (single-stage anodization) without (left) and with expansion in phosphoric acid (right), EPD parameters: 0.2 wt.%, 20°C, 340 rpm, 100 V, 90 min
Finally, the influence of hot water compaction at > 96 °C with the addition of a coating inhibitor on a highly ordered oxalic acid layer with compact particle intercalation (EPD parameter: 0.2 wt.% TiO2, 100 V, 90 min) was investigated. The SEM images show that the particles are dissolved out of the pore opening, whereby the photocatalytic activity of the surface comes to a standstill. After hot water compaction without a coating inhibitor, however, a reduced photocatalytic activity with an rPCE value of 4.6 can be measured. However, the SEM images also show the coating of pseudoboehmite AlO(OH), which must be prevented at this point. It is therefore necessary to examine the extent to which corrosion resistance is already present after particle incorporation and whether a compaction step can be dispensed with.
Corrosion tests
The following samples were examined with regard to their corrosion properties:
- Pre-treated aluminum sheet EN AW-5005 (degreased, pickled, decapped)
- Anodized sheet (oxalic acid anodization)
- Compacted oxalic acid anodizing layer
- Oxalic acid anodized layer with TiO2 particles
The non-anodized and only pre-treated AA5005 surface initially has a resting potential of around -0.27 V vs. SHE, which shifts to the more negative range to -0.4 V vs. SHE within 60 min. The anodized and partially compacted or impregnated surfaces show very different values at the start of the measurement, but all stabilize at a similar value of around -0.1 V vs. SHE over the course of the 60-minute measurement. At -0.05 V vs. SHE, the resting potential of the compacted anodization layer is slightly more positive than that of the other anodization layers. Overall, all anodized layers in the façade cleaner have a resting potential that is around 300 mV more positive than the non-anodized AA5005 surface (Fig. 11).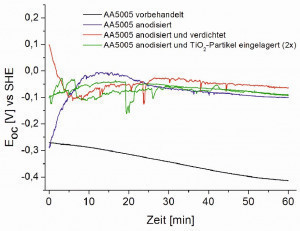 Fig. 11: Resting potential measurements in acidic Al and façade cleaner (pH value 1.8)
Fig. 11: Resting potential measurements in acidic Al and façade cleaner (pH value 1.8)
The clear protective effect of the anodized surfaces can be seen in the subsequent impedance measurements. In the low-frequency range, the samples anodized in oxalic acid with values >1 MΩcm2 have an impedance modulus at least two orders of magnitude higher than the non-anodized surface with 10 kΩcm2. The densification of the layer leads to a further increase in the impedance value compared to the non-densified layer, whereby the filling of the anodized layer with the TiO2 particles has a similar - in one case even stronger - effect than the usual densification process (Fig. 12).
The final potentiodynamic polarization measurements also confirm the clear protective effect of the anodization layers. The pre-treated but not anodized surface exhibits corrosion current densities of 1.5-2 µA/cm2. The application of the anodizing layers not only shifts the free corrosion potential by approx. 300 mV into the more positive range, but the corrosion current densities also drop into the nanoampere range (Fig. 13). The lowest corrosion current density is measured for the sample area of the layer filled with TiO2 particles, which showed the highest value in the low-frequency range in the impedance measurements. This area has the best barrier effect and therefore the best corrosion protection.
Overall, the tests demonstrate a very high corrosion resistance of the anodized and TiO2-modified coatings to the acidic façade cleaner.
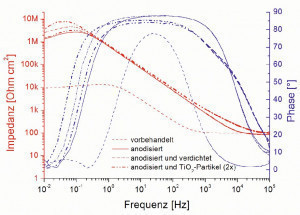 Fig. 12: Impedance spectra (Bode plots) of the differently treated AA5005 sheets after 60 minutes of resting potential measurement in acidic Al and façade cleaner (pH1.8).
Fig. 12: Impedance spectra (Bode plots) of the differently treated AA5005 sheets after 60 minutes of resting potential measurement in acidic Al and façade cleaner (pH1.8).
Conclusion
In the course of this work, highly structured, meso- and macroporous anodized surfaces were produced by means of oxalic acid anodization. The subsequent particle intercalation was carried out by means of electrophoretic intercalation from solvent-based systems. Depending on the EPD parameters, an intercalation result up to complete pore filling can be achieved in both highly structured and modified industrial anodized layers. The evaluation in accordance with ISO 22197-1 certifies that the filled, highly structured anodized coatings have a high photocatalytic activity with rPCE values of up to 12.8 and therefore clearly exceed the minimum requirement of ≥ 2. Clarification of the negative effect of hot water compression on the functionality of the anodized surface is still the subject of research and development work. The corrosion tests in an acidic aluminum and façade cleaner showed that the application of the anodized layers significantly increases the corrosion resistance of the aluminum material. The impedance measurements in particular made it clear that the introduction of TiO2 particles into the anodized pores can achieve a similar or even better barrier effect than the hot compaction of an unfilled layer. This means that subsequent hot sealing after functionalization of the layers could possibly be dispensed with, as this has a negative effect on the photocatalytic properties. However, further corrosion tests over longer periods of time are necessary in order to determine and compare the duration of the protective effect.
With regard to effectiveness, it must be mentioned that the NOx problem cannot be completely solved by photocatalytically functionalized surfaces alone. Calculations based on deposition rates predict a reduction in NOx values of a few percent (<10 %) [34]. This may seem low at first glance, but must be seen in relation to the effect of other measures. For example, a study on the effectiveness of low emission zones concludes that they achieve less than a 4 % reduction in NOx levels [35]. The photocatalytically active anodized surfaces can therefore be seen as a component of measures to reduce NOx levels in urban areas.
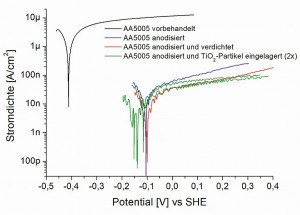 Fig. 13: Current density-potential curves of the differently treated AA5005 sheets in acidic Al and facade cleaner after resting potential and impedance measurements.
Fig. 13: Current density-potential curves of the differently treated AA5005 sheets in acidic Al and facade cleaner after resting potential and impedance measurements.
Acknowledgments
The authors would like to thank the German Federal Ministry of Economics and Climate Protection (BMWK) for funding the project via the German Federation of Industrial Research Associations (AiF) as part of the program for the promotion of joint industrial research (IGF) under No. 20136 N.
Literature
[1]Federal Environment Agency, https://www.umweltbundesamt.de/themen/luft/luftschadstoffe-im-ueberblick/stickstoffoxide, accessed on 25.05.2022
[2]World Health Organization. (2021). WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulphur dioxide and carbon monoxide. World Health Organization. https://apps.who.int/iris/handle/10665/345329
[3]Association of the Mineral Paints Industry, https://www.vdmi.de/de/produkte/angewandte-photokatalyse/produktspektrum/photoaktive-baustoffe/, retrieved on 25.05.2022
[4]C. Kammer: Aluminum Pocketbook. Vol. 1: Fundamentals and Materials, 15th edition, Aluminum-Verlag, Düsseldorf 1995
[5]A. Lotz: Studies on the nanostructuring of aluminum surfaces using electrochemical methods, Diss., University of Giessen, 2009
[6]M. Levering: Structuring of surfaces with nanoporous aluminum oxide, Diss., University of Duisburg-Essen, 2003
[7]S. Ono; M. Saito; H. Asoh: Self-ordering of anodic porous alumina formed in organic acid electro-lytes, Electrochim. Acta, 51, 5 (2005), 827-833
[8]J.E. Houser: Modeling the steady-state growth of porous anodic alumina, Diss., Iowa State UniVersity, 2008
[9]W. Hübne;, C.Th. Speiser: Die Praxis der anodischen Oxidation des Aluminiums, Aluminium Verlag Düsseldorf, 1978
[10]P. Wernick; R. Pinner; P.G. Sheasby: The surface treatment and Finishing of aluminum alloys, ASM International, 1987
[11]R. Mann, S. Hansal, W.E.G. Hansal: Nanoparticle incorporation in plasma-electrolytic oxidation, Int. J. Surf. Eng. Coat. 94, 3 (2016), 131-138
[12]S.K. Weidmann; W. Fürbeth; O. Yezerska; U. Sydow; M. Schneider: Modification of anodizing layers on aluminum materials by chemical nanotechnology, Galvanotechnik 101 (2010), 1728-1744
[13]B. Fori; P.-L. Taberna; L. Arurault; J.P. Bonino; C. Gazeau; P. Bares: Electrophoretic impregnation of porous anodic aluminum oxide film by silica, Colloids Surf. A 415, (2012), 187-194
[14]C. Kormann; D.W. Bahnemann; M.R. Hoffmann: Preparation and Characterization of Quantum-Size Titanium Dioxide (TiO2), J. Phys. Chem. 92, (1988), 5196-5201
[15]D.W. Bahnemann: Ultrasmall Metal Oxide Particles: Preparation, Photophysical Characterization and Photocatalytic Properties, Isr. J. Chem. 33, (1993), 115-136
[16]US Research Nanomaterials Inc, https://www.us-nano.com/inc/sdetail/484, retrieved on 25.05.2022
[17]Nyacol Nano Technologies Inc, https://www.nyacol.com/products/titanium-dioxide/, retrieved on 25.05.2022
[18]V. Etacheri; C.Di Valentin; J. Schneider; D. Bahnemann; S.C. Pillai: Visible-light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments, J. Photochem. Photobiol, C 25 (2015), 1-29
[19]C. Frantz; A. Lauria; C.V. Manzano; C. Guerra-Nuñez; M. Niederberger; C. Storrer; J. Michler; L. Philippe: Nonaqueous Sol-Gel Synthesis of Anatase Nanoparticles and Their Electrophoretic Deposition in Porous Alumina, Langmuir 33(43), 2017, 12404-12418
[20]Fachverband angewandte Photokatalyse: Evaluation of the NO degradation performance of photocatalytically active products, Technical Document, 2017
[21]Association of the Mineral Paints Industry, https://www.vdmi.de/de/produkte/angewandte-photokatalyse/freiw-selbstverpflichtung/, retrieved on 09.06.2022
[22]J.P. O'Sullivan; G.C. Wood: The morphology and mechanism of formation of porous anodic films on aluminum, Proc. R. Soc. Lond. A 317(1531), 1970, 511-543
[23]A.P. Li; F. Müller; A. Birner; K. Nielsch; U. Gösele: Hexagonal pore arrays with a 50-420 nm in-terpore distance formed by self-organization in anodic alumina, J. Appl. Phys. 84(11), 1998, 6023-6026
[24]T. Aerts; T. Dimogerontakis; I. de Graeve; J. Fransaer; H. Terryn: Influence of the anodizing tem-perature on the porosity and the mechanical properties of the porous anodic oxide film, Surf. Coat. Technol. 201(16-17), 2007, 7310-7317
[25]H. Masuda; K. Fukuda: Ordered metal nanohole arrays made by a two-step replication of honey-comb structures of anodic alumina, Science 268(5216), 1995, 1466-1468
[26]O. Jessensky: Self-Organized Formation of Hexagonal Pore Structures in Anodic Alumina, J. Elec-trochem. Soc. 145(11), 1998, 3735
[27]K. Nielsch; J. Choi; K. Schwirn; R.B. Wehrspohn; U. Gösele: Self-ordering Regimes of Porous Aluelf-ordering Regimes of Porous Alu-mina: The 10 Porosity Rule, Nano Lett. 2(7), 2002, 677-680
[28] H. Masuda; M. Satoh: Fabrication of Gold Nanodot Array Using Anodic Porous Alumina as an Evap-oration Mask, Jpn. J. Appl. Phys. 35, 1B, L126-L129, 1996
[29] T. Kikuchi; D. Nakajima; O. Nishinaga; S. Natsui; R. Suzuki: CNANO 2015, 11(5), 560-571
[30] W. Lee: Structural Engineering of Porous Anodic Aluminum Oxide (AAO) and Applications, in Springer series in materials science, Nanoporous Alumina, D. Losic and A. Santos, eds, Springer In-ternational Publishing, Cham, 2015, pp. 107-153
[31] S.J. Limmer; T.P. Chou; G.Z. Cao: A Study on the Influences of Processing Parameters on the Growth of Oxide Nanorod Arrays by Sol Electrophoretic Deposition, J. Sol-Gel Sci. Technol. 36, 2005, 183-195
[32] R.N. Basu; C.A. Randall; M.J. Mayo: Fabrication of Dense Zirconia Electrolyte Films for Tubular Solid Oxide Fuel Cells by Electrophoretic Deposition, J. Am. Ceram. Soc. 84(1), 2001, 33-40
[33] L. Besra; M. Liu, Progress in Materials Science 52(1), 2007, 1-61
[34] M. Gallus; R. Ciuraru; F. Mothes; V. Akylas; F. Barmpas; A. Beeldens; F. Bernard; E. Boonen; A. Boréave; M. Cazaunau; N. Charbonnel; H. Chen; V. Daële; Y. Dupart; C. Gaimoz; B. Grosselin; H. Herrmann; S. Ifang; R. Kurtenbach; M. Maille; I. Marjanovic; V. Michoud; A. Mellouki; K. Miet; N. Moussiopoulos; L. Poulain; P. Zapf; C. George; J.F. Doussin; J. Kleffmann: Photocatalytic abatement results from a model street canyon, Environ. Sci. Pollut. Res. 22, 2015, 18185-18196
[35] P. Morfeld; D.A. Groneberg; M.F. Spallek: Effectiveness of low emission zones: large scale analy-sis of changes in environmental NO2, NO and NOx concentrations in 17 German cities, PLoS One 9, 2014, e102999


