The project was able to gain new insights into the mechanisms of defect formation and differentiate between non-critical and defect-relevant process steps. A significant role of the interface between the casting material or casting skin and the copper plating in connection with the formation and temporary storage of hydrogen is indicated.
Motivation
The standard for the pre-treatment process for zinc die casting prior to electroplating is [3]:
- mild alkaline boiling degreasing, followed by rinsing
- electrolytic degreasing (cathodic1)) with a high silicate concentration, followed by rinsing
- fluoride-containing decapitation with slight attack on the alloy and minimal removal, followed by rinsing
- Galvanization and further coating sequence if necessary
1) Note: according to other sources, anodic degreasing is also possible(www.interzinc.org; retrieved 08.02.2016; also [4])
The choice of the correct decapping is of great importance and depends on the alloy composition. Fluorides are recommended for materials containing Pb and Si; otherwise a diluted sulphuric acid is sufficient [SCH1982]. Under no circumstances should non-ferrous metals be dissolved in the pre-treatment media, as metals more noble than zinc contaminate the surface through cementation.
For decorative components, a typical coating sequence consists of
- Copper (cyanide)
- Copper (acidic)
- Semi-bright nickel
- Bright nickel, possibly alternatives such as satin nickel or pearlescent nickel
- Chrome
The undercoats are used for good adhesion, levelling and to optimize corrosion protection, while the top coats are used to create the visual impression. Purely functional zinc die-cast components are not galvanized, but passivated directly; depending on the visual appearance and corrosion protection requirements, galvanizing is followed by passivation. This makes it possible to match the appearance of combined galvanized steel parts.
Objective and procedure
The aim of the project was to investigate the various processes upstream of the galvanic coating of zinc die castings, including the casting process during production and downstream treatment steps and ageing processes, in order to derive optimized process instructions and make the complex and error-prone overall process more robust. The alloys were selected in consultation with the project committee, in which a number of casting manufacturers and companies from the electroplating and surface technology sectors were represented. In addition to the variation of casting parameters, the investigations included the use of release agents and variations in mechanical and chemical pre-treatment. The investigations focused on the interaction between the ageing processes and the coatability. By incorporating the latest, cyanide-free electrolyte developments, the galvanic layer sequence was deliberately critically controlled in order to test the robustness of an optimized pre-treatment in the comprehensive characterization (corrosion, adhesion, defects).
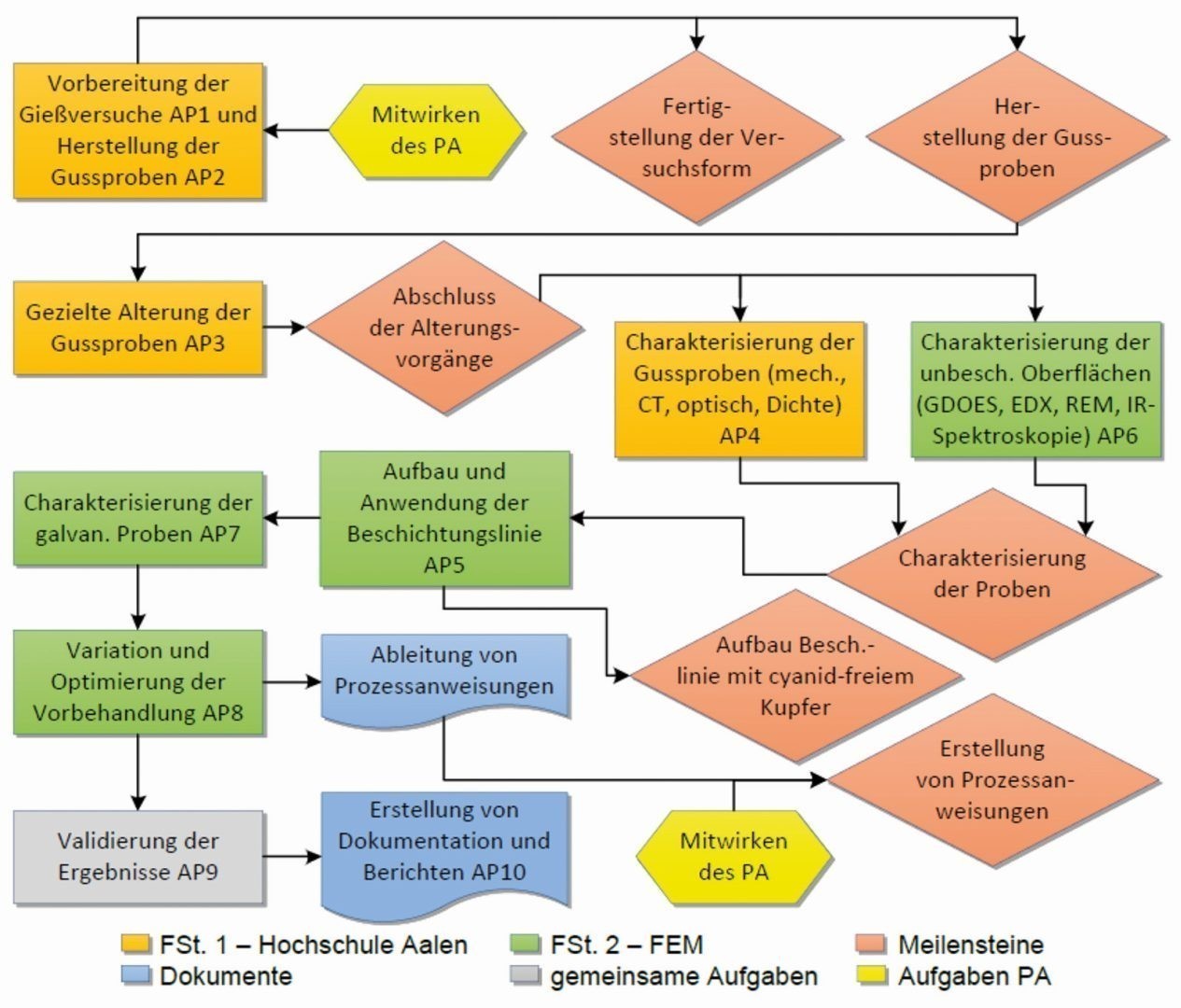 Fig. 1: Project management plan with process coordination to illustrate the interactions of the work packages
Fig. 1: Project management plan with process coordination to illustrate the interactions of the work packages
The wall thickness of the component, the mold temperature and the gate speed were varied as important casting parameters. Fluctuations in the mold temperature can have a strong effect on the formation of the casting skin, especially on the formation of cold flow areas and porosities (gas inclusions and blowholes). In addition to the variation of casting parameters, the investigations also included the use of different release agents. The influence of the release agent on the coatability and its possible interaction with the electroplating materials has not yet been investigated. Wall thickness and casting conditions can also have a considerable influence on gases trapped near the surface, which in turn can lead to rejects. Further influencing factors are post-treatments of the components such as blasting, vibratory grinding, thermal deburring, grinding and polishing. The extent to which post-treatment changes the surface and thus influences the subsequent coating was investigated.
 Fig. 2: Field allocation of the casting sample surfaceForprecise timing of the entire process chain for trouble-free surface coating, the two research departments, Foundry Technology at the HTW Aalen and the Electroplating Department of the fem in Schwäbisch Gmünd, worked closely together and investigated the complex interrelationships in order to derive an optimum process description for reliable production with the highest quality. The procedure followed the diagram in Figure 1.
Fig. 2: Field allocation of the casting sample surfaceForprecise timing of the entire process chain for trouble-free surface coating, the two research departments, Foundry Technology at the HTW Aalen and the Electroplating Department of the fem in Schwäbisch Gmünd, worked closely together and investigated the complex interrelationships in order to derive an optimum process description for reliable production with the highest quality. The procedure followed the diagram in Figure 1.| 1 | Degreaser SLOTOCLEAN AK 340 (T: 65 °C, t: 15 min.) |
| 2 | Flow rinse city water (1 min.), flow rinse deionized water (15s) |
| 3 |
Cathodic degreasing:
electrolytic degreaser SLOTOCLEAN EL 130 (T: 50 °C, t: 30 s, i: 4 A/dm2)
or alternatively
anodic degreasing:
electrolytic degreaser SLOTOCLEAN EL 130 (T: 30 °C, t: 15 s, i: 1.5 A/dm2) |
| 4 | Flow rinse city water (1 min.), flow rinse deionized water (15 s) |
| 5 | Activation SLOTOCLEAN DECASEL 5 (T: 30 °C, t: 15 s) |
| 6 | City water flow rinse (1 min.), deionized water flow rinse (15 s) |
| 7 |
Cyanide copper electrolyte CUPRUM 10 (T: 60 °C, t: 15 min., i: 3 A/dm2)
or alternatively
a cyanide-free copper electrolyte CUPA SANCY HE3, Chemopur H. Brand GmbH (T: 45 °C, t: 60 min., i: 1 A/dm2) |
| 8 | Flow rinse city water (1 min.), flow rinse deionized water (15 s) |
| 9 | Nickel electrolyte Nickel sulphamate bath MS (T: 50 °C, t: 15 min., i: 5 A/dm2) |
| 10 | Flow rinse city water (1 min.), flow rinse deionized water (15 s) |


