Due to the long operating times required for plug connections, these are pre-treated with contact lubricants. As an alternative to this, silver dispersion coatings with various dry lubricants as dispersoids were examined for their suitability to increase the wear resistance of current-carrying plug connections.
Introduction
The trend towards miniaturization in electrical engineering and the resulting increase in performance poses an increasing challenge, particularly for the coating of current-carrying plug connections. Connectors with a high number of mating or friction cycles are often pre-treated with a contact lubricant in order to minimize friction wear during operation. In order to achieve the required operating times of several decades in some cases, the contact lubricant must be designed for long-term stability and temperature resistance. For these reasons, fluorochemicals are usually used as lubricants, which are critical both in terms of production and disposal.
As an alternative to the commonly used contact lubricants, silver dispersion coatings with various dry lubricants as dispersoids were examined under the influence of increased temperature to determine their suitability for increasing the wear resistance of current-carrying plug connections. Based on previously published test results [1], pure silver, silver-MoS2, silver-WS2 coatings and silver-graphite systems were aged in long-term tests at near-application temperatures (140 °C and 180 °C). To validate the silver dispersion layers, their friction and wear properties as well as hardness and microstructure were analyzed. A special feature of this test series is the deliberate omission of a diffusion barrier between the silver dispersion layer and the base material.
Experimental results
The aging tests were carried out on two different sample types. Unsupported films were produced for the material characterization. For the tribological tests, planar brass substrates were used, which were cyanide pre-copper-plated and pre-silver-plated. In order to investigate the influence of the dispersoids on the diffusion behavior of zinc from the base material or of copper from the copper interlayer into the silver dispersion layers, a nickel interlayer was deliberately omitted in the test series. The samples used in this study are listed in Table 1 . Further details on the preparation of the samples can be found in [1].
|
Sample |
Electrolyte |
i [A/dm2] |
t [min] |
d [µm] |
Particle type |
Particle size D50 [µm] |
cparticle [g/L] |
|
Pure silver |
Cyan. Ag |
1 |
12,5 |
6 |
- |
- |
- |
|
Silver Graphite |
graphite |
4,5 |
120 |
||||
|
Silver - WS2 |
WS2 |
3 |
10 |
||||
|
Silver - MoS2 |
MoS2 |
8,5 |
10 |
To assess the topographical changes initiated by the long-term test, selected samples were examined in detail using scanning electron microscopy (Gemini SEM 300 from Zeiss). The chemical composition of the layers was determined using energy dispersive X-ray spectroscopy (EDX).
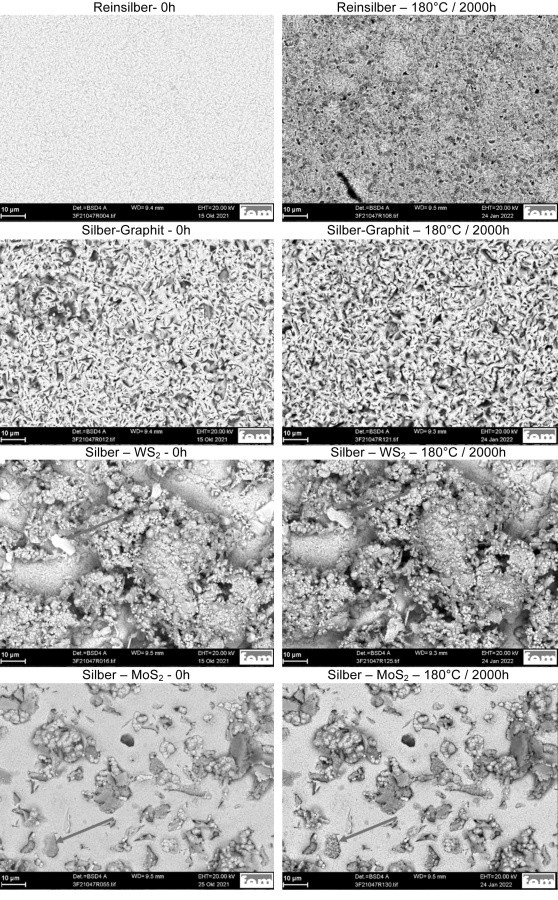 Fig. 1: SEM images of the sample surface before and after aging at 180°C for 2000h (pure silver, silver-graphite, silver-WS2, silver-MoS2)
Fig. 1: SEM images of the sample surface before and after aging at 180°C for 2000h (pure silver, silver-graphite, silver-WS2, silver-MoS2)
The tribological properties were determined by means of a pin-on-disk test using a "CSEM Pin On Disk Tester" tribometer and a 100Cr6 ball coated with pure silver as a counter body at a normal force of 1 N and a sliding speed of 52.4 mm/s. The temperature for the pin-on-disk test was 23 °C at a relative humidity of 50 %.
To evaluate the coating hardness, instrumented indentation tests were carried out on the various coatings in the metallographic section (Fischerscope HM500). The maximum test load was 30 mN. The indenter was a Vickers indenter.
Results
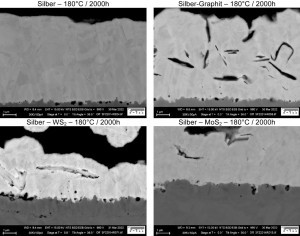 Fig. 2: Cross-sectional micrographs after ageing ' at 180°C for 2000 h (pure silver, silver graphite, silver WS2, silver MoS2)The scanning electron microscopic examinations show that the elements copper and oxygen, and in some samples also zinc, can be detected in the near-surface area on all samples examined after an ageing time of 500 h at 140 °C. However, an actual change in the surface topography at 140°C is only observed on the silver MoS2 samples after 2000h. From this it can be concluded that the diffusing species have not yet completely penetrated the silver layer in the pure silver layers and the silver-WS2 and silver-graphite dispersion layers. At an aging temperature of 180 °C, changes in the surface structure can be observed in all samples. Figure 1 compares the surface topography of the different samples in the initial state and after 2000 h at 180 °C aging using backscatter detector images. The interaction between backscattering intensity and average atomic number makes it possible to detect changes in the elemental composition of the surface.
Fig. 2: Cross-sectional micrographs after ageing ' at 180°C for 2000 h (pure silver, silver graphite, silver WS2, silver MoS2)The scanning electron microscopic examinations show that the elements copper and oxygen, and in some samples also zinc, can be detected in the near-surface area on all samples examined after an ageing time of 500 h at 140 °C. However, an actual change in the surface topography at 140°C is only observed on the silver MoS2 samples after 2000h. From this it can be concluded that the diffusing species have not yet completely penetrated the silver layer in the pure silver layers and the silver-WS2 and silver-graphite dispersion layers. At an aging temperature of 180 °C, changes in the surface structure can be observed in all samples. Figure 1 compares the surface topography of the different samples in the initial state and after 2000 h at 180 °C aging using backscatter detector images. The interaction between backscattering intensity and average atomic number makes it possible to detect changes in the elemental composition of the surface.
The pure silver and silver-graphite samples show the greatest change in backscatter intensity. In comparison, only local changes can be observed in the silver-WS2 and silver-MoS2 samples, particularly in the area of the dispersoids protruding from the surface (see markings).
Figure 2 shows cross-sectional images of the pure silver and silver dispersion layers after aging at 180 °C for 2000 hours. It can be seen that the structure of the silver matrix of the silver dispersion layers Silver-WS2 and SilverMoS2 is strongly disturbed in the vicinity of the incorporated dispersoids. Increased concentrations of the diffusing species copper and zinc are detected at these points, especially in the area of the grain boundaries.
Figure 3 shows the mean friction coefficients of both pure silver and the silver dispersion layers before and after aging at 180 °C for 2000 h. The tests on pure silver coatings serve as a reference. All of the silver dispersion layers examined show good tribological properties compared to the pure silver layer, even after ageing at 140 °C or 180 °C. The heat treatments have little to no influence on the average coefficient of friction of the silver dispersion layers.
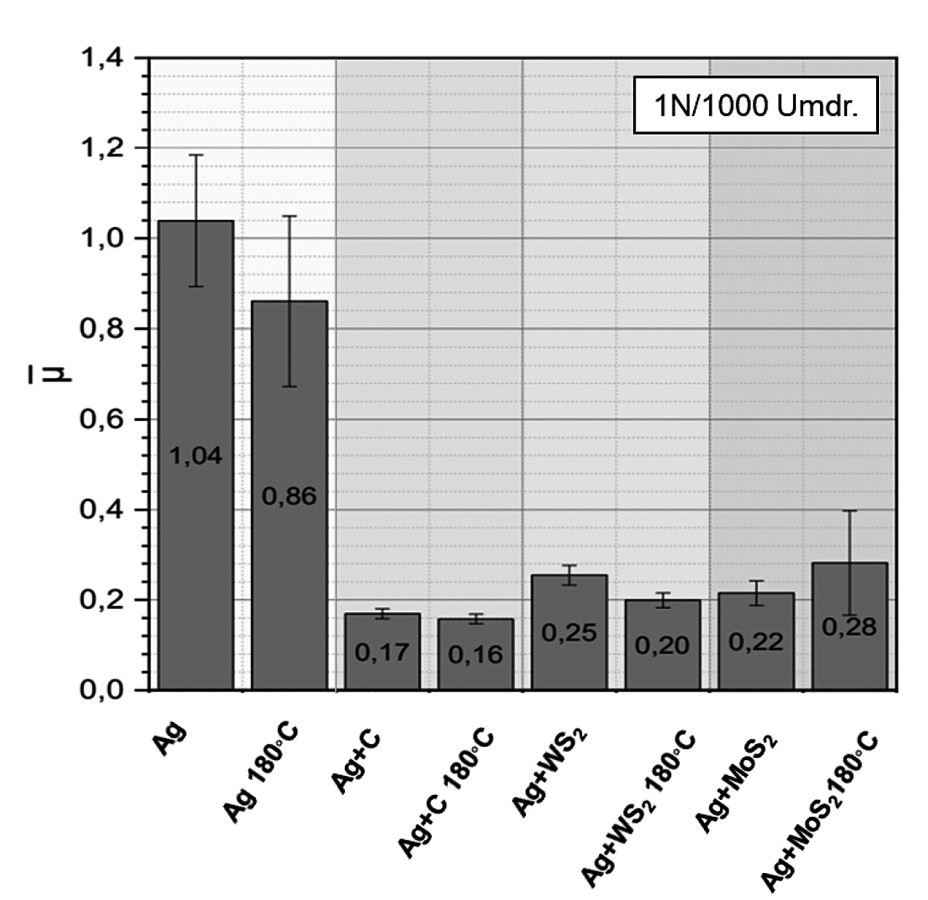 Fig. 3: Friction coefficient of the respective silver dispersion layer in comparison before and after ageing at 180 °C for 2000 h
Fig. 3: Friction coefficient of the respective silver dispersion layer in comparison before and after ageing at 180 °C for 2000 h
The results of the instrumented indentation tests show a slight decrease in the coating hardness in the initial state as a result of the incorporation of the various dispersoids. Furthermore, a decrease in hardness is registered for all systems during the ageing process (see Figs. 4 and 5). The pure silver system exhibits the greatest change in hardness with a decrease in hardness from approx. 105 HV to approx. 60 HV at 140 °C and 180 °C after 100 h. The change in hardness in the silver-graphite system is less pronounced; the values fall from the initial value of 96 HV to 74 HV after 100 h at 140 °C and 180 °C respectively. After further aging at 140 °C, the values remain constant, whereas the hardness drops to 66 HV at 180 °C. The behavior of the silver-MoS2 layers is similar to that of the silver-graphite layers. Starting at just under 100 HV, the hardness drops to 77 HV after 100 h, and to approx. 70 HV with even longer ageing. With silver-WS2, the drop in hardness is somewhat less pronounced (from 93 HV to 80 HV after 100 h or until the end of ageing). The observed drop in hardness is known for silver coatings and is usually caused by grain growth as a result of heat treatment. The presence of dispersoid particles appears to reduce this process. Since the hardness values of the dispersion layers, at least for the silver-C and silver-WS2 systems, have not yet reached a plateau value after 1000 hours of ageing at 180 °C, a further drop in hardness cannot be ruled out with prolonged thermal stress.
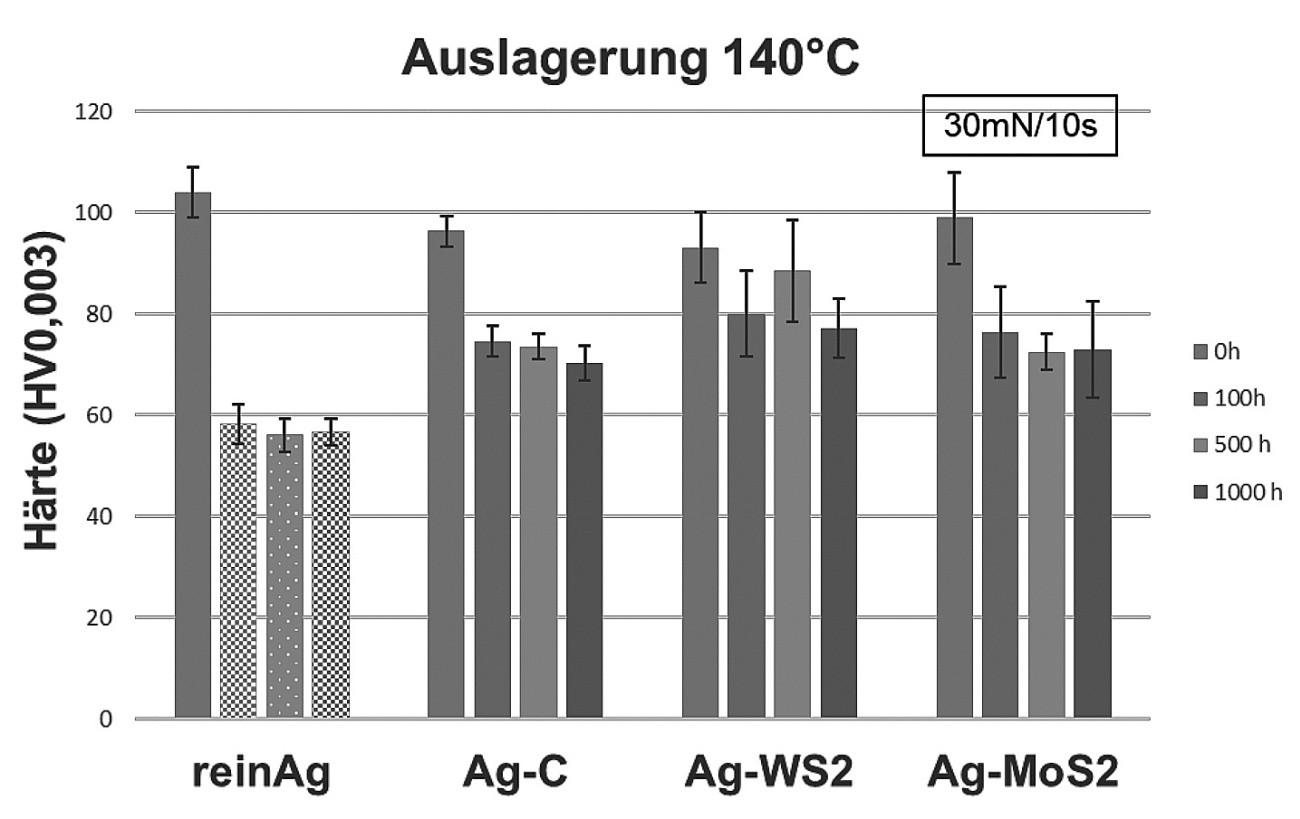 Fig. 4: Hardness values of the respective coating system at the beginning, after 100 h, 500 h and 1000 h of ageing at 140 °C
Fig. 4: Hardness values of the respective coating system at the beginning, after 100 h, 500 h and 1000 h of ageing at 140 °C
Summary
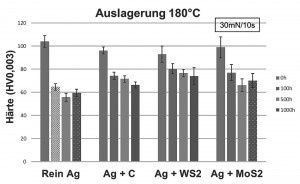 Fig. 5: Hardness values of the respective coating system at the beginning, after 100 h, 500 h and 1000 h of ageing at 180 °CInseveral tests, silver dispersion coatings with differently embedded dispersoids (graphite, WoS2, MoS2) were exposed to elevated temperatures in a long-term test. The test results show that there is generally a good bond between silver and the dispersoids used. The silver-graphite, silver-MoS2 and silver-WS2 systems stand out in comparison to pure silver coatings due to their good tribological properties. These systems exhibit a relatively low coefficient of friction both before and after thermal aging. The incorporation of dispersoids slows down the decrease in hardness as a result of heat treatment compared to pure silver coatings. In almost all systems, the diffusion of copper from the applied intermediate layer or of zinc from the base material to the coating surface could be detected. Only the silver-graphite system showed a slower diffusion of the elements copper and zinc.
Fig. 5: Hardness values of the respective coating system at the beginning, after 100 h, 500 h and 1000 h of ageing at 180 °CInseveral tests, silver dispersion coatings with differently embedded dispersoids (graphite, WoS2, MoS2) were exposed to elevated temperatures in a long-term test. The test results show that there is generally a good bond between silver and the dispersoids used. The silver-graphite, silver-MoS2 and silver-WS2 systems stand out in comparison to pure silver coatings due to their good tribological properties. These systems exhibit a relatively low coefficient of friction both before and after thermal aging. The incorporation of dispersoids slows down the decrease in hardness as a result of heat treatment compared to pure silver coatings. In almost all systems, the diffusion of copper from the applied intermediate layer or of zinc from the base material to the coating surface could be detected. Only the silver-graphite system showed a slower diffusion of the elements copper and zinc.
Literature
[1] Arnet, R.; Egetenmeyer, A.; Kappl, H.; Willing, H.: Silver dispersion coatings with self-lubricating properties, Galvanotechnik 112 (2021) 1, p. 21 ff


