The REACh chemicals regulation has significantly tightened the parameter requirements for substances and mixtures since 2007. The regulations, which are monitored by the European Chemicals Agency ECHA in Helsinki, have resulted in a tsunami of information requirements, tasks and costs for companies that manufacture, process, import and transfer chemicals into their supply chains [1]. Implementation of the REACh requirements is still patchy - but the EU Commission already sees a need for further action in chemicals legislation.
Why REACh?
The European chemicals legislation REACh [2] (Registration, Evaluation and Authorization of Chemicals) is based on experience in the EU member states. However, it was once the responsibility of the authorities to check the safety of chemicals. Very little substance-specific data was available on chemicals that were traded on the market before 1981. The authorities had to request missing data from manufacturers and importers if they became aware of environmental and health hazards. Despite the adoption of a "Responsible Care" pledge [3], a commitment to "responsible action", this was also very cumbersome in Germany. The European Parliament and the Commission in Brussels had to act, also in view of numerous accidents involving chemicals (see box on page 306).
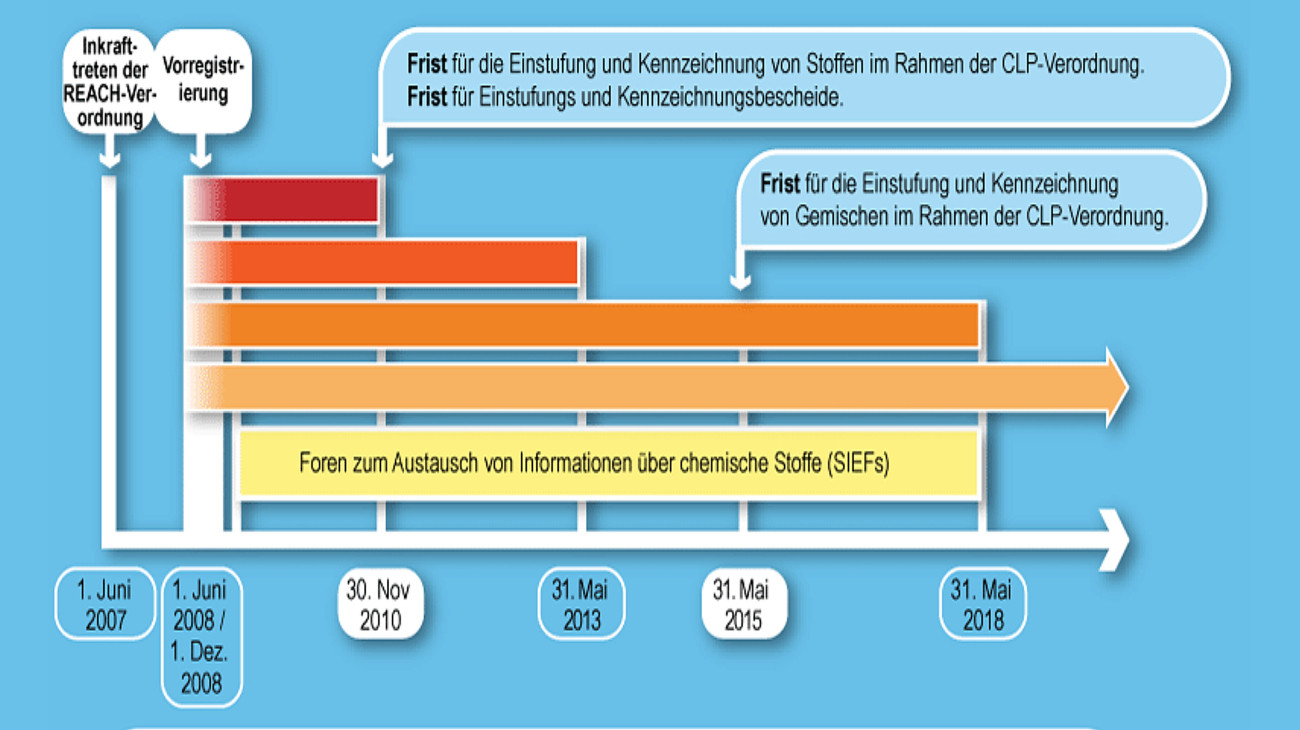 Fig. 1: Implementation plan for REACh and CLP (Graphic: Wolfgang Hasenpusch)
Fig. 1: Implementation plan for REACh and CLP (Graphic: Wolfgang Hasenpusch)
After lengthy discussions with industry associations and the entry into force of the European REACh Regulation in 2007, which no longer required national regulation, chemical manufacturers and importers in Europe had to apply for a mandatory authorization procedure for their substances and mixtures according to a fixed schedule, depending on the volume band of chemicals to be placed on the market (Fig. 1). In doing so, they must determine all relevant substance data and assess the risks posed by the substances themselves (Fig. 2). No chemical may be placed on the market within the EU without authorization from a specially created authority in Helsinki, the European Chemicals Agency ECHA [4]. The transfer in the supply chains is also regulated, specified in Germany since 2023 by the "Lieferketten-Sorgfaltspflichten-Gesetz" [5]. The replacement of particularly hazardous chemicals with less hazardous substances or other processes enjoys state support. The REACh Regulation also gives consumers the right to receive information about chemicals in products [6].
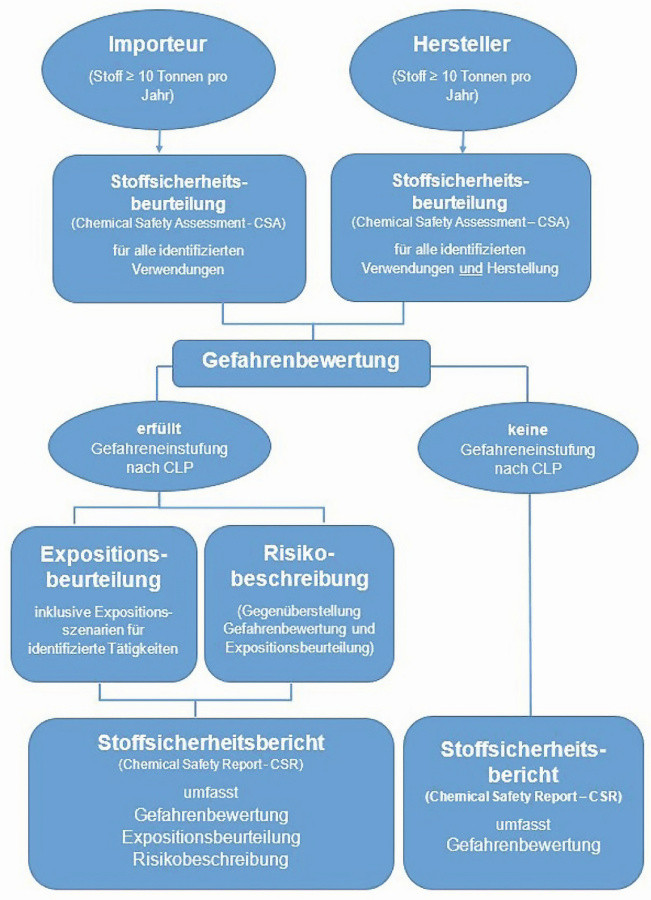 Fig. 2: Flow chart for substance classification according to REACh (diagram: Wolfgang Hasenpusch)
Fig. 2: Flow chart for substance classification according to REACh (diagram: Wolfgang Hasenpusch)
The REACh implementation
In the past registration phases, European chemical companies and importers have succeeded in registering over 30,000 substances with the ECHA with extensive information on their properties. By overlapping the chemicals of manufacturers and importers, ECHA received over 100,000 applications for authorization for the further use and distribution of their substances.
As early as 2007, ECHA published a first guideline on the implementation of REACh [7], which included 115 pages on data transfer using the IUCLID (International Uniform Chemical Database) software program. Many more were to follow on specific topics. In 2017, for example, ECHA published a guideline on how to clearly characterize substances [8]. These are:
- Substance designation and name in the so-called IUPAC nomenclature
- Information on the molecular and structural formula
- composition.
In 2020, the ECHA issued guidelines for the preparation of safety data sheets (Fig. 3) in version 4.0 with 146 pages [9]. The extended safety data sheet, eSDS, also contains "exposure scenarios". If suppliers do not support the use of chemicals in their own company and do not create exposure scenarios, they should check whether they have an obligation to create a chemical safety report that includes corresponding exposure scenarios for their own uses of the substances [10].
 Fig. 3: Content of the extended safety data sheet
Fig. 3: Content of the extended safety data sheet
Users who use hazardous substances registered in accordance with REACh in quantities of more than 10 tons per year should receive an extended SDS with exposure scenarios from suppliers. If use or conditions of use are not covered in any of the exposure scenarios provided by suppliers, the following options are available:
- The supplier includes the use/conditions of use of their own company
- The described use is adapted in the supplier's own company
- The process is discontinued or carried out alternatively
- Search for another supplier who can provide the relevant exposure scenarios
- Carry out your own chemical safety assessment [11].
Shortcomings in REACh implementation
Even during the first few years, the industry associations and ECHA found that many companies were completely unable to meet the stringent requirements for their chemicals (Fig. 4).
 Fig. 4: "There are new directives for your company again!" (Drawing: Wolfgang Hasenpusch)
Fig. 4: "There are new directives for your company again!" (Drawing: Wolfgang Hasenpusch)
New parameters for the commodity chemicals had to be determined, countless association meetings and consultations were held. Special institutes had to be found for toxicological tests, which were soon overloaded. Toxicologists, who had previously found it difficult to find a job after graduating, were suddenly in demand like never before.
Consortia made it easier to obtain data and reduced the high costs involved. Nevertheless, even safety data sheets are still very incomplete in some cases [12], (Fig. 5). It seems incomprehensible that the most rudimentary data are not obtained from the literature or estimated by means of correlations, e.g. by interpolating and extrapolating known data:
- Interpolations and extrapolations of known analogous substances
- Relationships between molecular weight and density
- Relationships between density and refractive index
- Relationship between boiling point and flash point
- Relationships between electrical and thermal conductivity.
 Fig. 5: Example of an SDS for synephrine, section 9.1As early as2019, the ECHA and the European Commission in Brussels recognized that the quality of the REACh safety dossiers submitted to date needed to be significantly improved [13]. They published a joint action plan listing missing information and requirements and agreed to carry out more reviews.
Fig. 5: Example of an SDS for synephrine, section 9.1As early as2019, the ECHA and the European Commission in Brussels recognized that the quality of the REACh safety dossiers submitted to date needed to be significantly improved [13]. They published a joint action plan listing missing information and requirements and agreed to carry out more reviews.
By 2023, ECHA intends to review the dossiers in the tonnage band above 100 metric tons per year (tpy) and by 2027 those in the tonnage band from 1 to 100 tpy [14]. However, numerous evaluations and authorizations are still missing. ECHA has imposed restrictions on the use of a number of substances. Furthermore, "safety data sheets" and "safety dossiers" need to be revised and supplemented.
The European industry associations support their member companies in the preparation of the extensive chemical safety files with their "EU REACh Dossier Improvement" [15, 16]. It contains suggestions on how companies can create, review and adapt their dossiers.
The European Chemical Industry Council (CEFIC) has presented its second report on the sector action plan for reviewing and updating REACh registration dossiers. According to the report, the implementation of the "REACh Dossier Improvement Action Plan" made good progress in 2020. European chemical and pharmaceutical companies reviewed around 2,731 REACh dossiers in 2019. The industry thus continued to work actively on improving the quality of registration dossiers [17].
Further requirements for REACh and GHS/CLP
In autumn 2020, the EU Commission published its "Chemicals Strategy for Sustainability" with numerous health and environmental protection measures as part of the European "Green Deal". This will once again result in far-reaching changes. Proposals to amend the CLP Regulation (Regulation on Classification, Labeling and Packaging of Substances and Mixtures) were already available at the end of 2022. The new version of the European REACh Regulation is planned for the end of 2023, for which many other environmental and occupational health and safety regulations will have to be adapted.
The European Commission's chemicals strategy pursues a regulatory approach that is very much based on the intrinsically hazardous properties of chemicals and mixtures, with no consideration given to safe activity and handling. This puts elements and their compounds from half the periodic table in the "dock". The EU Commission is planning new data requirements, restrictions on use and comprehensive regulation of groups of substances with certain properties, such as persistence, mobility or endocrine disruptors (hormone-like substances).
In future, restrictions on chemicals in consumer products or in professional applications will often be imposed without prior risk assessment and consultation with manufacturers in a fast-track procedure. Certain polymers are to be subject to registration. The introduction of an assessment factor for possible combination effects of substances is also being examined.
New hazard classes are to be introduced under CLP, in some cases irrespective of whether these are hazard characteristics. The chemicals strategy also introduces new terms such as "safe and sustainable chemicals", "substances of concern" or "essential uses". Clear definitions are needed here, which take into account the practical implications. If the planned chemicals strategy is implemented unchanged, this will have at least two serious consequences:
- High adaptation and conversion costs and
- Limited diversity of substances.
According to initial estimates, up to a third of European chemical production would be affected by the introduction of new hazard classes and restrictions on use. Interest groups, such as the German Chemical Industry Association (VCI) and the German Metals Federation (WV Metalle), are therefore proposing amendments in coordination with the European industry organizations CEFIC (European Chemical Industry Council) and EUROMETAUX (European non-ferrous metals association). These include both the new definitions and criteria as well as the future procedure for risk management when handling substances and mixtures.
All European industry associations are committed to
- Stable legal framework conditions and planning security
- Low-risk use of substances throughout their life cycle
- Retention of the risk-based approach under REACh
- Maintaining global GHS/CLP harmonization without national solo efforts
- Sustainable linking of innovations with the requirements of chemicals legislation [18].
The Zentralverband Oberflächentechnik e.V. (ZVO) also fears a disproportionate tightening of chemicals legislation, particularly in the departure from the risk-based approach to regulation and strict regulations on the handling of substance groups [19].
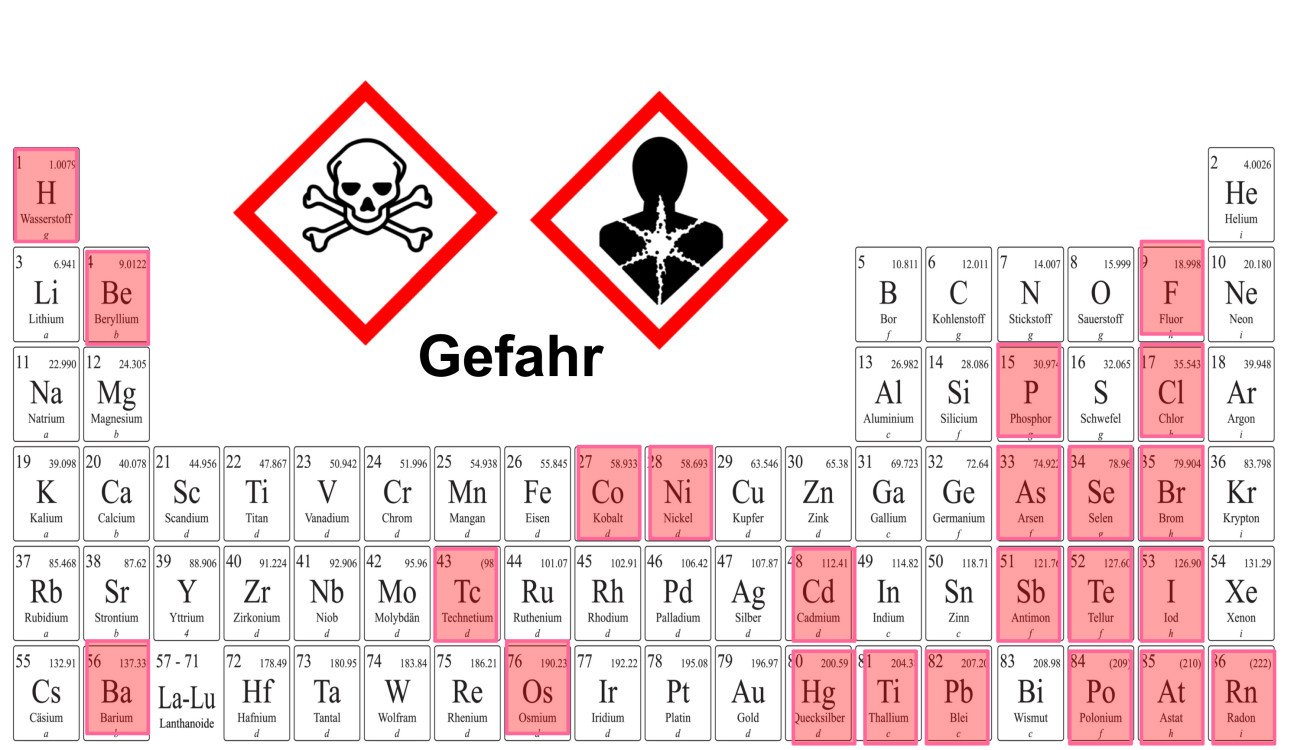 Fig. 6: Toxic elements in the periodic table, highlighted in pink, excluding lanthanides and actinides
Fig. 6: Toxic elements in the periodic table, highlighted in pink, excluding lanthanides and actinides
The exclusive and general focus on the intrinsic properties of hazardous substances is also seen as fatal. Cyanides, for example, are undoubtedly acutely and chronically toxic substances that should be replaced as far as possible. Appropriate protective measures should also apply when handling them. However, it would make no sense to ban them. Numerous metals and non-metals would be removed from the periodic table of elements alone if their industrial use, let alone that of their compounds, were to be banned (Fig. 6).
What's next for REACh?
Back in September 2020, EU parliamentarians, ECHA representatives and industry associations discussed the future of further measures in the implementation of the REACh Regulation [20].
While industry representatives pointed out how much effort, resources and money companies in the EU have invested in REACh implementation so far, politicians and authorities pointed out existing gaps and weaknesses in the systems. There was a consensus that the authorities do not monitor enough and that existing control mechanisms do not function adequately.
The EU Commission is of the opinion that the existing REACh regulations do not yet meet all requirements for the protection of citizens and the environment. The level of harmful substances in products is still too high. Many hazardous substances in the environment are also continuing to increase.
The chemical associations never tire of arguing that this is not about the hazardousness of chemicals, but that the legislator must consider hazardousness together with handling and exposure. In particular, there is the problem of the non-harmonized approach of the current 27 member states. Therefore, the desire for a defined procedure for REACh implementation controls as well as for the punishment of violations dominates.
For the EU Commission, chemicals policy is an important point for the protection of health and the environment, in which industry also plays a central role in relation to the "European Green Deal" strategy, which is about sustainability andCO2 neutrality, among other things.
Nevertheless, the EU Commission sees ample need for action to take further regulatory measures. In particular, the adoption of chemical safety according to REACh by countries such as the UK, Turkey, Korea and others is spurring on the ambition of the EU Commission to play a pioneering role and take further steps. Buzzwords such as "REACh Plus" and "REACh 2.0" are already making their rounds in Brussels.
In the EU Commission and the EU Parliament, the focus is often on activism and not on economic realities. It is therefore essential for companies to coordinate with each other, to send expert representatives to the association committees or even to Brussels, so that the extensive expertise in chemicals legislation can take concerted action.
This applies to the management of chemicals as well as occupational health and safety, the environment, hazardous goods and emergency management. Competent audits in the companies can show where there are still legal uncertainties.
INFO
 Self-commitment, ha! - Now we say what chemical safety means! (Drawing: Wolfgang Hasenpusch)REACh = RACHE
Self-commitment, ha! - Now we say what chemical safety means! (Drawing: Wolfgang Hasenpusch)REACh = RACHE
Could the word REACh be a corruption of the word revenge?
That would not be too far-fetched. After all, the industry, primarily the chemical industry, had long tried to put the issue of "chemical safety" on the back burner with "Responsible Care" and voluntary commitments.
After numerous incidents, such as the accidents at Höchst-Farbwerke in Frankfurt in the 1990s and a number of serious chemical accidents [21], the European Commission was forced to take tougher action:
- 1969: Fish kill in the Middle Rhine due to endosulfan
- 1970: Naphtalene fire at the Erkner tar plant
- 1970: Explosion of a petrol tank at the Minol tank farm in Magdeburg-Rothensee
- 1974: Flixborough accident, England, explosion of cyclohexane
- 1974: Explosion in ethanol plant, CSSR
- 1976: Accident in Seveso due to dioxins, Italy
- 1978: Explosion at the Sayda gas compressor station
- 1982: Explosion in the gas purification plant at the "Schwarze Pumpe" chemical plant
- 1984: Disaster in Bhopal, India, involving methyl isocyanate
- 1986: Major fire at Sandoz, Switzerland
- 1987: Hydrogen explosion at Boxberg power plant
- 1992: Fire at Allied Colloids, England, caused by azobis(isobutyronitrile)
- 1996: Vinyl chloride fire in Schönebeck
- 1997: Fuel fire in Elsterwerda
- 1998: Dam failure of a tailings lagoon containing heavy metals in Aznalcóllar, Spain
- 2000: Dam burst in Baia Mare, Romania, with cyanide tailings
- 2001: Explosion of ammonium chloride in Toulouse, France,
Literature
[1] Hasenpusch, W.: "REACh- und GHS/ CLP-Verordnung - Zwei europäische Mammut-Regelwerke halten die Chemische Industrie in Atem, CLB, Verl. Rubikon, Gaiberg, 61/07-08 (2010) 338-345
[2] EU-REACh Chemicals Regulation (EC) No. 1907/2006: Entry into force: June 1, 2007
[3] Responsible Care = "responsible action": Initiative of the chemical industry since 1991, independent of legal requirements, to create continuous improvements in companies regarding environmental protection, product responsibility, occupational health and safety, plant safety and hazard prevention, transport safety as well as dialog, renewed trust and acceptance
[4] European Chemicals Agency, ECHA, an EU authority under Regulation No. 1907/2006 of 18 December 2006: Regulation of technical, scientific and administrative aspects of the registration, evaluation and authorization of chemicals
[5] Supply Chain Due Diligence Act, LkSG; entry into force: January 1, 2023
[6] https://www.umweltbundesamt.de/themen/chemikalien/reach-chemikalien-reach
[7] ECHA: Guidance on the implementation of REACh (2007)
[8] ECHA: Guidance on the identification and naming of substances according to REACh and CLP, Version 2.1, May (2017); Annex VI, 2
[9] ECHA: Guidance on the compilation of safety data sheets, version 4.0 - December 2020
[10] https://www.dguv.de/ifa/fachinfos/reach/sicherheitsdatenblatt/erweitertes-sicherheitsdatenblatt-(esdb)/index.jsp
[11] ECHA: Practice Guidance 13: Handling Exposure Scenarios - Guidance for Downstream Users, ECHA-12-G-04-EN, 6 (2012)
[12] https://www.extrasynthese.com/MSDS/ALL/1591.pdf
[13] REACh Evaluation Joint Action Plan (2019): As early as 2018, the German Federal Institute for Risk Assessment, BfR, and the German Federal Environment Institute, UBA, found that only one third of the 3,800 REACh dossiers met the legal requirements
[14] https://the-ncec.com/de/regulatory-compliance/uk-and-eu-reach/eureach-dossier-improvement
[15] https://cefic.org/policy-matters/reach-dossier-improvement-action-plan/
[16] https://echa.europa.eu/de/support/how-to-improve-your-dossier-to-be-deleted
[17] https://www.vci.de/themen/chemikaliensicherheit/reach/ueberpruefung-von-registrierungsdossiers-durch-unternehmen-geht-voran-2-bericht-reach-dossier-improvement-action-plan.jsp
[18] Hanschmidt, A.: VCI position compact EU chemicals strategy, VCI, Frankfurt, 9.1.2023
[19] ZVO: "Tightening of the REACh Regulation planned", ZVOreport, Central Association of Surface Technology e. V., Hilden, 1 (2023) 22
[20] https://www.umco.de/de/blog/artikel/REACH-und-Green-Deal-Chemikalienpolitik-der-Zukunft.html
[21] https://www.google.com/search?q=chemie-unfaelle


