The AAACoatcoating produced using sol-gel technology can improve and extend the property profile of electroplated and anodized coatings. The glass-like, silicon dioxide-based coating achieves electrical insulation with high dielectric strength, further improves corrosion protection and significantly increases the decorative variety. The chemical-, UV- and weather-resistant hydrophobic surface can be further modified by inorganic functional pigments with regard to sliding behavior, slip protection, etc.
Electroplated coatings are produced by the electrochemical deposition of metallic deposits on substrates in an electrolytic bath. Depending on the metal or alloy, wear-resistant (chrome, nickel), decorative (gold, silver) and/or corrosion-protective (zinc) coatings can be achieved. However, the high electrical conductivity (electrical protection) is a disadvantage in many applications. Sufficient corrosion protection usually requires thick layers, which is associated with high material and power consumption. The aesthetic appearance is determined and limited by the choice of coating metal. Anodized layers on aluminium are produced by anodic oxidation in a mostly acidic (sulphuric acid, oxalic acid) electrolyte. The hard, porous and transparent layers can be further modified by organic or electrolytic coloring [1] (Fig. 1). In the latter case, however, the color palette is reduced to bronze tones. The weather resistance of organically colored facades is usually not given and the chemical resistance in the alkaline range is limited for all anodized coatings.
Sol-Gel/ AAACoatcoating
AAACoatcoating can avoid or reduce some of the disadvantages listed above. AAACoatis a glass-like, nanoceramic coating based on sol-gel technology. It is produced in a process in which selected monomers containing silicon oxide are formed into a silicon dioxide network by hydrolysis and subsequent condensation, with the release of water, which has a special property profile [2, 3] (Fig. 2).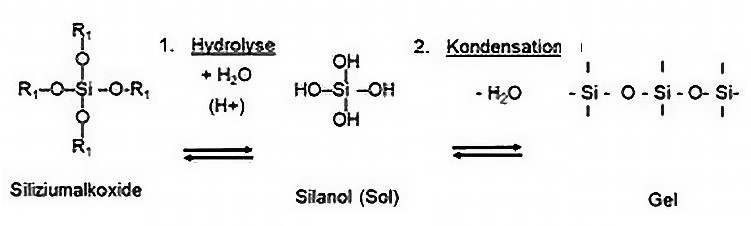 Fig. 2: The sol-gel process (example Si-based)
Fig. 2: The sol-gel process (example Si-based)
AAACoatis produced in an alcoholic solution and can be applied to the substrate like an organic coating system by dipping (dip coating), spraying, rolling or continuously in a coil coating process. Due to its thin consistency, even fine pores, cracks or cavities can be coated. After application, the sol-gel coated parts must be cross-linked at ≥ 150°C.
Brief property profile of the AAACoatcoating
Due to its inorganic structure and its similarity to amorphous glass, AAACoathas very good weather and UV resistance as well as high temperature resistance (>500 °C). The coating is highly transparent, resistant to practically all solvents such as alcohols, petrol, acetone, MEK 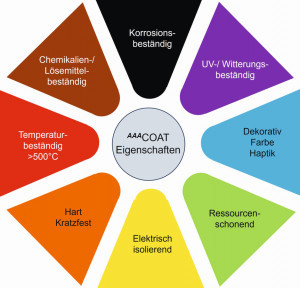 Fig. 3: Property profile of AAACoat/ sol-gel and very resistant in diluted acids. In the alkaline range, protection against cleaning agents up to pH 12 is provided and far exceeds the possibilities of pure anodized coatings, for example. AAACoatcoatings are hard and brittle and can be applied in layer thicknesses of >0.5 µm to approx. 15 µm. With some special pigmentations, even significantly thicker layers of up to >50 µm can be achieved (Fig. 3). By adding decorative and functional pigments, the property profile of the sol-gel binder (matrix) can be further varied. Inorganic, corrosion-resistant color pigments enhance the visual appearance without reducing properties such as temperature or weather resistance. Sliding properties (dry lubrication) are sustainably improved with sliding pigments such as molybdenum disulphide (MoS2) or graphite, and slip resistance is significantly increased with wear-resistant particles such as silicon carbide, diamond, etc.
Fig. 3: Property profile of AAACoat/ sol-gel and very resistant in diluted acids. In the alkaline range, protection against cleaning agents up to pH 12 is provided and far exceeds the possibilities of pure anodized coatings, for example. AAACoatcoatings are hard and brittle and can be applied in layer thicknesses of >0.5 µm to approx. 15 µm. With some special pigmentations, even significantly thicker layers of up to >50 µm can be achieved (Fig. 3). By adding decorative and functional pigments, the property profile of the sol-gel binder (matrix) can be further varied. Inorganic, corrosion-resistant color pigments enhance the visual appearance without reducing properties such as temperature or weather resistance. Sliding properties (dry lubrication) are sustainably improved with sliding pigments such as molybdenum disulphide (MoS2) or graphite, and slip resistance is significantly increased with wear-resistant particles such as silicon carbide, diamond, etc.
Differences between AAACoatand organic coating systems
Although sol-gel coating solutions are applied in a similar way to organic coating systems, AAACoatdiffers from conventional coating systems in key properties (Table 1).
|
Properties of |
organic |
AAACoat/Sol-Gel |
Remarks |
|
Main chemical elements |
C,O,N,H |
Si,O |
Compare in each case |
|
Temperature resistance |
<100°C |
>500°C |
|
|
Behavior in case of fire |
Smoke development |
No smoke development |
|
|
typical layer thicknesses |
15-500µm |
0.5-15µm |
|
|
Hardness, scratch resistance |
low |
high |
|
|
UV-/ |
tends to embrittlement |
very good |
|
|
Solvent resistance |
partially |
very good |
1)Organic paint binders such as alkyd resins, synthetic resins, oils, etc. no silicones!
Tab. 1: Differences between organic lacquer systems and AAACoat
AAACoaton electroplated surfaces
In order to achieve the necessary corrosion protection on steel or other substrates, several or thicker layers usually have to be applied during galvanic treatment. In addition, there may be exposed areas in cavities, edges or cracks (hard chrome). The thin consistency of the coating covers these uncoated areas. The capillary effect leads to reliable filling of the exposed zones, particularly in the case of cracks or crevices (Fig. 4). In many applications, even a thin top coat is sufficient to significantly increase corrosion resistance. The combination of electroplating and sol-gel coating leads to material savings in many cases. On the electroplated metal surfaces, AAACoatlayers insulate the substrate electrically and prevent or suppress contact corrosion (bimetallic corrosion). The hydrophobic surface also repels moisture. In addition, the thin sol-gel layer increases the corrosion resistance. Figure 5 shows a galvanized body panel on a copper plate in direct contact after 24 hours in a slightly alkaline, 5% NaCl solution (pH~ 10). Galvanized body panel and copper plate on the left side are coated with approx. 2-3 µm AAACoat. Body panel and copper plate on the right side are uncoated.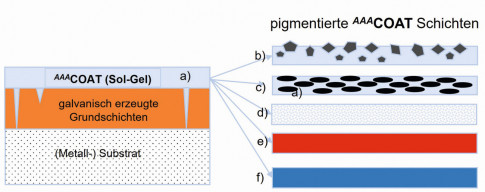 Fig. 4: AAACoat as top layer on electroplated layers - with and without pigments: a) without pigmentation b) hard material particles (SiC, diamond...) c) lubricating pigments (MoS2, graphite...) d) matting agent, e) and f) color pigments
Fig. 4: AAACoat as top layer on electroplated layers - with and without pigments: a) without pigmentation b) hard material particles (SiC, diamond...) c) lubricating pigments (MoS2, graphite...) d) matting agent, e) and f) color pigments
 Fig.5: Galvanic separation by AAACoat. Left coated, right uncoated (bimetallic corrosion).
Fig.5: Galvanic separation by AAACoat. Left coated, right uncoated (bimetallic corrosion).
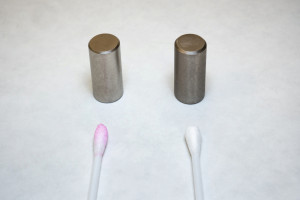
Sol-gel layers can also serve as protection against tempering colors. On nickel, copper, brass, etc. Coatings with this coating prevent or reduce tarnishing of the metals at higher temperatures. Nickel-plated and chrome-plated parts trigger skin allergies in many people. When aggressive sweat comes into contact with nickel or chrome, the corresponding ions are released, which can lead to serious inflammation. The thin coating with sol-gel (approx. 2-3µm) prevents the release of metal ions. A simple nickel test available in pharmacies with a cotton swab (soaked in dimethylglyoxime) in an alkaline environment (ammonia) leads to red coloration when the corresponding Ni ions are dissolved out (Fig. 6a, 6b).
 Fig. 6a: Bright chrome-plated (front) and nickel-plated (back) furniture knobs. The two furniture knobs on the right-hand side are coated with 2-3µm AAACoat. The furniture knob at the front is finished with matt AAACoat. The sharp mirror images of the two bright chrome-plated furniture knobs show that the highly transparent sol-gel coating does not result in any loss of shine
Fig. 6a: Bright chrome-plated (front) and nickel-plated (back) furniture knobs. The two furniture knobs on the right-hand side are coated with 2-3µm AAACoat. The furniture knob at the front is finished with matt AAACoat. The sharp mirror images of the two bright chrome-plated furniture knobs show that the highly transparent sol-gel coating does not result in any loss of shine
With the help of functional pigments in the submicron (< 1 µm) or nanometer range (< 0.1 µm), sol-gel coatings can be further optimized in many ways. Sliding particles such as molybdenum disulphide (MoS2) or graphite can be used to create surfaces with low friction coefficients and low wear. We see great potential in forming tools for plastics. Thanks to the high temperature resistance of the AAACoat matrixand the lubricating pigments, various plastics can be formed at several 100 °C without adhesion (Fig. 7a). On the other hand, the inclusion of hard and angular pigments such as diamond, silicon carbide (SiC) etc. in the sol-gel matrix leads to surfaces with a very high coefficient of friction. The latter can be used as anti-slip protection (Fig. 7b).
AAACoaton anodized surfaces
Anodized layers are transparent aluminium oxide layers that have a hexagonal structure with a thin barrier layer (Fig. 8, bottom right). The pores in the upper part of these cells can be filled with soluble dyes and then sealed in a bath of boiling water (hot water sealing) or with nickel salts (cold sealing). In hot water sealing, a swelling reaction occurs with the water and an aluminum oxide-hydroxide layer is formed, which closes the pores and increases the corrosion resistance. In cold sealing, the pores are closed by the formation of nickel/aluminium complexes at lower temperatures.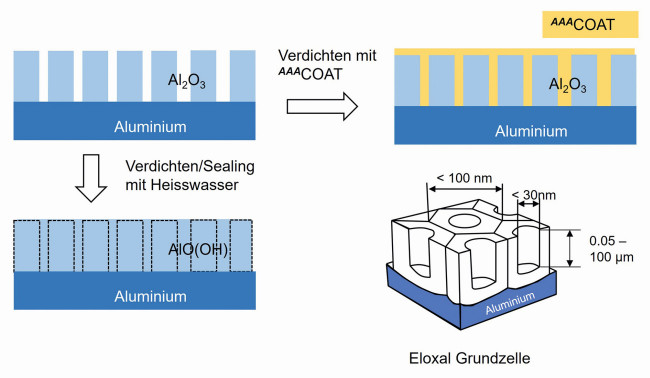 Fig.8: Schematic representation of anodized aluminium. Top left: uncompacted anodization layer, bottom left: Hot water compacted, top right: anodized with AAACoat, bottom right: hexagonal base cell of the anodized layer
Fig.8: Schematic representation of anodized aluminium. Top left: uncompacted anodization layer, bottom left: Hot water compacted, top right: anodized with AAACoat, bottom right: hexagonal base cell of the anodized layer
Internal and external edges, small bending radii and cavities are critical in anodizing treatment. As two thirds of the anodized layer grows into the aluminium, material is missing from edges and small bending radii. These areas therefore remain uncoated. Cavities such as the inside of pipes are largely cut off from the current supply due to the Faraday effect and cannot form a layer. The thin liquid consistency of AAACoatfills the gaps, among other things. This process is further enhanced by capillary effects. AAACoateven penetrates into the narrow pores of sulphuric acid anodized layers and thus leads to additional anchoring of the sol-gel layer. This also explains the very good adhesion of sol-gel to practically all anodized aluminium alloys. Depending on the application, AAACoatlayers of 2-15 µm in thickness are applied to the anodized layer (Fig. 9).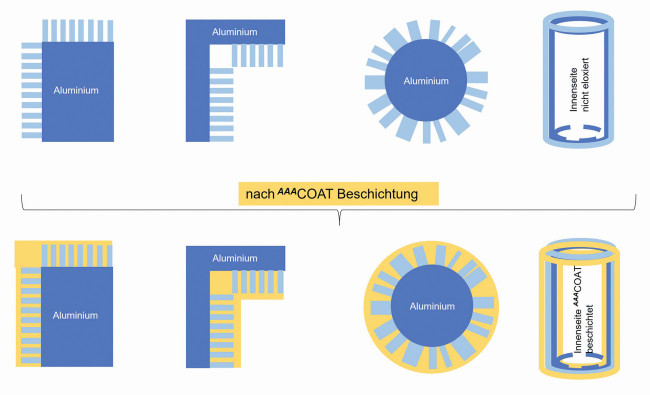 Fig. 9: Critical zones after anodizing aluminium. Corners, edges, small curves and cavities lead to exposed areas that can be closed with AAACoat
Fig. 9: Critical zones after anodizing aluminium. Corners, edges, small curves and cavities lead to exposed areas that can be closed with AAACoat
Figure 10 shows various aluminium profiles that have been anodized to a thickness of approx. 22 µm, partially dip coated in blue and half coated with AAACoat. At approx. 24 µm, the total thickness of the sol-gel coated part is only 2 µm thicker than the pure anodized coating. However, the dielectric strength [4] of the coated part (approx. 2000 V (DC)) is around twice as high as that of the anodized-only part (approx. 1000 V). By increasing the sol-gel coating, the dielectric strength can be significantly increased (up to over 3000 V). AAACoatalso promotes the temperature stability of the anodized coating. While pure conventional anodized coatings drastically lose their dielectric strength after a temperature treatment of around 190 °C, the sol-gel-gelled coating almost retains its insulating properties [5]. The inside of cavities can also be coated well using this process. A quick test in an alkaline environment clearly demonstrates the protective effect of a thin AAACoat-coatedand colored anodized layer. An approx. 10 µm thick anodized and red coloured shiny aluminium trim (Al 98.85) was exposed to an alkaline medical cleaner at 40 °C for 15 hours, whereby one of the trim parts was additionally protected with an approx. 1-2 µm thick AAACoat layer. While the gloss of the surface of the anodized sample has completely disappeared and some of the paint has dissolved in the medical cleaner, the AAACoat-coatedsample shows no difference to the initial state (Fig. 11).
The hydrophobic properties of AAACoatsol-gel coatings greatly reduce the adhesion of dirt or other contaminants. In contrast to conventional anodized surfaces, sprayed-on traces of graffiti can be easily removed with alcohol or other solvents. AAACoatoffers a wide range of decorative design options. Virtually all combinations of glossy or matt and the entire color spectrum are possible. In particular, surfaces can be created that cannot be produced with anodized paints, such as white. In addition, no or lower requirements are placed on the surface quality of the aluminum alloy for color-covering coating with sol-gel. In many cases, anodizing quality (aluminium with reduced iron and silicon impurities) can be dispensed with, which reduces the procurement costs of the component to be coated.
Outside of electroplating and anodizing
AAACoatcan also be applied directly to parts that have not previously been electroplated or anodized. In this way, the property profile of metal, glass, ceramic, etc. materials is further improved by a thin sol-gel coating. Typical examples are
- Anti-fingerprint and non-stick coatings on stainless steel
- Non-stick coatings directly on metals (aluminum, steel, copper, brass, magnesium...)
- Scratch-resistant coatings on polycarbonate (PC)
- Water-repellent coating on paper or cardboard
- Coloring, transparent coatings on glass
- Sealing of fired ceramics (bricks...)
Special features of sol-gel coating
Before applying a sol-gel coating to a new substrate for the first time, a preliminary test is advisable. Experience shows that properties such as adhesion, flow behavior, etc. can vary depending on the surface condition of the galvanically applied layer. Depending on the surface, this must be activated in advance and the sol-gel coating system adapted to the process. Sol-gel coatings are generally not completely gas-tight. The protective effect on the substrate is based on a complex interaction between the base material and the coating. Components coated with AAACoatare simply stripped with organic solvents such as alcohol etc. when not yet baked. In the cross-linked, baked form, the component can be stripped with concentrated alkaline solutions such as sodium hydroxide (NaOH) at a slightly elevated temperature. The application potential of AAACoat/Sol-Gel for electroplated and anodized coatings is briefly summarized in Table 2.
|
Optimization |
Electroplated |
AAACoat/sol-gel |
Remarks |
|
electrical insulation |
- all metals |
all AAACoat |
with the exception of high metal and graphene |
|
Electrical dielectric strength |
- anodized aluminium |
non-pigmented |
Increase EDF to |
|
Coating of |
- all metals |
all AAACoat |
with sol-gel dip |
|
Edge and |
- Hard chrome, anodizing |
all AAACoat |
|
|
Thermal |
- Nickel, copper, brass |
all AAACoat |
|
|
Corrosion protection |
- all metals |
all AAACoat |
especially for thin metal coatings |
|
transparent |
- silver, copper |
non-pigmented |
|
|
Decorative |
- all metals |
pigmented AAACoat |
Variation of color (incl. white and black), gloss, feel, etc. possible |
|
Protection against |
- Nickel, chrome |
All AAACoat |
|
|
Antifingerprint / |
- all metals |
all AAACoat |
Table 2: Optimization potential of AAACoat coatings - Summary
INFO
Fuchs Materials & Engineering (FME GmbH)
develops, produces and distributes AAACoat (sol-gel), coats on a contract basis and also offers consulting and development for functional and decorative surfaces as well as corrosion protection.
Literature
[1] S. Wernick, R. P. (1987). The Surface Treatment and Finishing of Aluminum and its alloys. Teddington, Middlesex, England: Finishing Publications LTD.
[2] C. J. Brinker and G. W. Scherer. (1990). Sol-gel science: the physics and chemistry of sol-gel processing, Academic Press, Boston, 1990. Boston: Academic Press.
[3] Schmidt, H. K. (2001). The sol-gel process: Inorganic synthesis methods . Chemie in unserer Zeit (35), 176-184.
[4] EN ISO 2376 (2010). Anodizing of aluminium and aluminium alloys - Determination of the electrical breakdown test.
[5] Fuchs, R. (04/2020). Electrical properties (dielectric strength) of anodized coatings. Surfaces POLYSURFACES surface treatment, 13-15.