Of the six platinum group metals platinum, palladium, iridium, rhodium and ruthenium, the hard, brittle, steel-blue osmium is characterized by exceptional properties: the pure transition metal of the 8th group of the periodic table has the highest compression modulus and the highest density of all elements. Due to its high price, its technical applications are limited to applications where extreme hardness and corrosion resistance are required. Separation from the other metals and most analytical methods are carried out by oxidative distillation of osmium(VIII) oxide, OsO4. Most derivatives are derived from this compound, whose thermodynamically and kinetically stable properties have led to numerous chemical and spectroscopic investigations.
1 The precious metal osmium
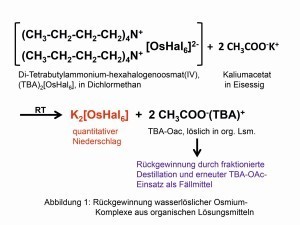 Fig. 1: Recovery of water-soluble osmium complexes from organic solventsThemetal with the highest density of 22.59 g/cm3 at room temperature (RT) was discovered by the later Cambridge professor Smithson Tennant (1761-1815) as early as 1804 together with iridium in the insoluble residue after the digestion of platinum in aqua regia:
Fig. 1: Recovery of water-soluble osmium complexes from organic solventsThemetal with the highest density of 22.59 g/cm3 at room temperature (RT) was discovered by the later Cambridge professor Smithson Tennant (1761-1815) as early as 1804 together with iridium in the insoluble residue after the digestion of platinum in aqua regia:
Pt(Os, Ir) + 8 HCl + 2 HNO3 → H2[PtCl6] + (Os, Ir) + 2 NOCl + 4 H2O
Osmium owes its name to the rettich-like odor (osmē, Greek = "smell, stench") of its volatile tetroxide, OsO4, which is present in low concentrations [1].
Osmium occurs together with the other platinum group metals (PGMs). Its concentration in the earth's crust is quoted at 0.0015 ppm, which is slightly higher than the metals platinum, iridium and gold.
There are currently around 500 kg of osmium in enrichment and production processes worldwide. Of the estimated 9m3 of this precious metal to date, only around 2m3 can be extracted as an element. That would be 44 tons with a current value of around 48 billion euros. Thus, 10,000 tons of PGM ore must be processed in many steps to finally obtain one ounce, 31.1 g, of osmium metal, with the dimensions of a sugar cube [2].
By 2017, a total of more than 200 deposits of solid osmium and its varieties with platinum and iridium had been identified worldwide [3].
However, the recycling of industrial osmium waste is of minor importance. It is produced at the end of the PGM separation process, where the metals osmium and ruthenium dissolve in a peroxide melt and are then converted into the volatile tetroxides with chlorine or sodium chlorate, NaClO3.
 Fig. 2: Pure osmium metal produced by transport reaction with chlorine Some nickel, chromium, copper and iron ore deposits in Russia, Canada or South Africa contain osmium in very small quantities. It is also found in sediments such as sand or gravel, for example in Russia, Ethiopia, Colombia or Borneo. The world's largest mining site is Witwatersrand in South Africa. Large reserves can be found in Turkey and Bulgaria. As only the osmium tetroxide dissolves as osmat(VI) in alcoholic sodium hydroxide solution, it can be separated from ruthenium:
Fig. 2: Pure osmium metal produced by transport reaction with chlorine Some nickel, chromium, copper and iron ore deposits in Russia, Canada or South Africa contain osmium in very small quantities. It is also found in sediments such as sand or gravel, for example in Russia, Ethiopia, Colombia or Borneo. The world's largest mining site is Witwatersrand in South Africa. Large reserves can be found in Turkey and Bulgaria. As only the osmium tetroxide dissolves as osmat(VI) in alcoholic sodium hydroxide solution, it can be separated from ruthenium:
Os(XIII)O4 + 2 NaOH + CH3-CH2-OH → Na2Os(VI)O4 + CH3-CH=O+ H2O
There are several ways to produce the pure osmium metal: On the one hand, quantitative precipitations with ammonium chloride or after reaction with hydrochloric acid and large organic ammonium cations are possible from the osmate(VI):
A. Na2OsO4 + 4 NH4Cl→ [OsO2(NH3)4]Cl2 + 2 NaCl + 2 H2O
B. Na2OsO4 + 10 HCl → H2[OsCl6] + Cl2 + 2 NaCl + 4 H2O
H2[Os(IV)Cl6] + NR4 → (R4N)2[OsCl6].
Poorly soluble hexahalogeno-osmates(IV) can be prepared with various onium cations, such as tetrabutylammonium (TBA), organic diammonium compounds or alkali metal cations in macrocyclic crown ethers [4].
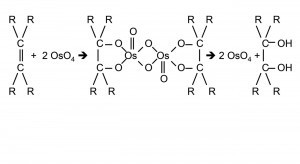
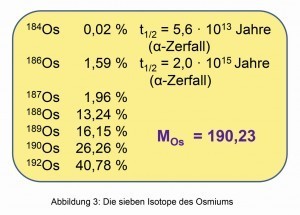 Fig. 3: The seven isotopes of osmiumThepoorly soluble hexahalogeno complexes with organic ammonium cations can be converted back into water-soluble compounds via "reciprocal precipitation" in organic solvents using organic alkali metal salts (Fig. 1). This method put an end to the destruction of countless nujol and tablet preparations for the purpose of spectroscopic investigations [4].
Fig. 3: The seven isotopes of osmiumThepoorly soluble hexahalogeno complexes with organic ammonium cations can be converted back into water-soluble compounds via "reciprocal precipitation" in organic solvents using organic alkali metal salts (Fig. 1). This method put an end to the destruction of countless nujol and tablet preparations for the purpose of spectroscopic investigations [4].
High-purity osmium, for example, is accessible via transport reactions in a halogen atmosphere (Fig. 2). The boiling point of osmium tetrachloride, OsCl4, is already 450 °C.
The molecular weight of osmium of 190.23 g/mol results from seven isotopes, five of which are stable. The radioactive decay of the isotopes Os-184 and -186, which are present in low concentrations, is characterized by very long half-lives (Fig. 3).
The world market for osmium is manageable. If you were to shape the almost 100 kilograms of osmium traded worldwide each year into a ball, it would be no bigger than a handball due to the high density of the element. It is primarily traded as a processed compound, above all in the form of osmium tetroxide in sealed glass ampoules [5].
2 A metal with exceptional properties
The pure metal is characterized by an intense steel-blue metallic luster. Osmium is the element with the highest density of all naturally occurring elements, just ahead of iridium. On both sides of the periodic table, the densities of the neighboring metals decrease (Fig. 4).
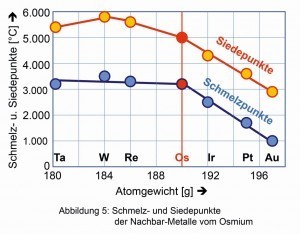
Among the PGMs, osmium has the highest melting and boiling points, but is trumped by its neighbors rhenium, tungsten and tantalum (Fig. 5).
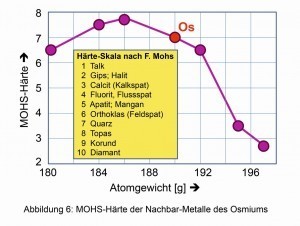
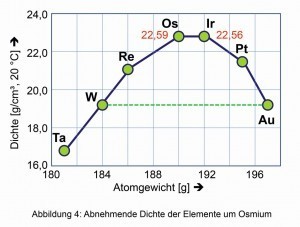 Fig. 4: Decreasing density of the elements around osmiumSimilarapplies to the comparative hardness derived from certain minerals according to the German mineralogist Friedrich Mohs (1773-1839) (Fig. 6). The compression modulus of 462 Giga-Pascal, the spontaneous elastic change in volume and thus density when isostatic pressure is applied, is also one of the highest of all known elements and compounds. Even the hard diamond is somewhat easier to compress at 443 GPa.
Fig. 4: Decreasing density of the elements around osmiumSimilarapplies to the comparative hardness derived from certain minerals according to the German mineralogist Friedrich Mohs (1773-1839) (Fig. 6). The compression modulus of 462 Giga-Pascal, the spontaneous elastic change in volume and thus density when isostatic pressure is applied, is also one of the highest of all known elements and compounds. Even the hard diamond is somewhat easier to compress at 443 GPa.
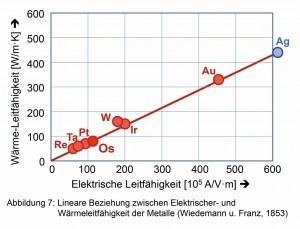
Thermal and electrical conductivity are nowhere near the values of gold and silver, but are close to platinum in these properties. The empirical "Wiedemann-Franz law" [6], which is based on the linear relationship between thermal and electrical conductivity, is also well fulfilled (Fig. 7).
As a precious metal, osmium reacts relatively sluggishly to digestion chemicals. Even aqua regia (HCl/HNO3-3:1) is hardly able to dissolve pure osmium. Only very strong oxidizing agents such as concentrated nitric acid, hot sulphuric acid or alkaline oxidation melts such as sodium peroxide and potassium chlorate attack osmium.
Finely dispersed metal, such as chemically precipitated osmium powder, spreads an odor similar to chlorine in the air and also causes halo effects in vision, such as when swimming for an hour in an indoor pool without swimming goggles.
Osmium tetroxide and all osmium compounds are considered very toxic. They can lead to pulmonary edema and cause skin and eye damage. The corresponding occupational exposure limit value, AGW, is therefore extremely low at 0.002 mg/m3.
The self-ignition and even self-explosion of chemically precipitated osmium powder should also not be forgotten. Like ruthenium powder, it is capable of storing over 1,600 volume parts of hydrogen per volume part of metal powder from the reduction chemicals, especially sodium boranate, NaBH4, and can therefore have an extremely explosive effect if the powder has not been degassed at over 600 °C in a nitrogen stream.
3 Uses of osmium
The range of applications is limited to a few technical areas due to the difficulty of processing and the high price. Gone are the days when brittle and costly to manufacture lamps were fitted with osmium and tungsten filaments and, thanks to the Osram company, have been lighting up streets and living rooms since 1905. Today, special alloys, use as catalysts as well as jewelry processing and value investment are among the main areas of use.
3.1 Alloys
 Fig. 8: Osmium ring (5 g) and tip of a fountain pen made from an osmium alloy Due to the high toxicity of the oxides, osmium is rarely used in its pure state in technical applications. Hard osmium-containing alloys of the platinum metals are occasionally used in abrasive and wearing processes, such as writing balls in ballpoint pens, exclusive fountain pen tips (Fig. 8), phonographic scanning needles, shafts and pins in instrument making and electrical contacts (Fig. 9), for example as iridium-osmium alloys, a combination that also occurs in nature [7-9].
Fig. 8: Osmium ring (5 g) and tip of a fountain pen made from an osmium alloy Due to the high toxicity of the oxides, osmium is rarely used in its pure state in technical applications. Hard osmium-containing alloys of the platinum metals are occasionally used in abrasive and wearing processes, such as writing balls in ballpoint pens, exclusive fountain pen tips (Fig. 8), phonographic scanning needles, shafts and pins in instrument making and electrical contacts (Fig. 9), for example as iridium-osmium alloys, a combination that also occurs in nature [7-9].
An alloy of 90 % platinum and 10 % osmium can be found in medical implants and artificial heart valves as well as in pacemakers [1].
3.2 Catalysis
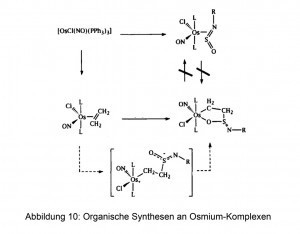
Osmium can also be used as a powerful catalyst in the reaction of gases. Hydrogenation catalysts made of osmium alloys are in use. Some osmium compounds in the form of solids or solutions serve as catalysts for the synthesis of fine chemicals and organometallic preparations (Fig. 10).
Dihydroxylation reactions have long been used in organic chemistry (Fig. 11). One of the best known is the so-called "Upjohn dihydroxylation" with catalytically used osmium tetroxide, OsO4, and N-methylmorpholine-N-oxide(NMO). The stereoselective "Sharpless dihydroxylation", named after its discoverer Barry Sharpless, was awarded the Nobel Prize in Chemistry in 2001 [10-12].
3.3 Jewelry and value investment
For a long time, the marketing of osmium metal was comparatively dormant when compared with the importance of other precious metals. Osmium powders and sponges, such as those produced in the separation veins of the refineries since their discovery in 1804, released clearly audible, unpleasant smelling and very toxic OsO4 gases on their large surfaces through reaction with atmospheric oxygen.
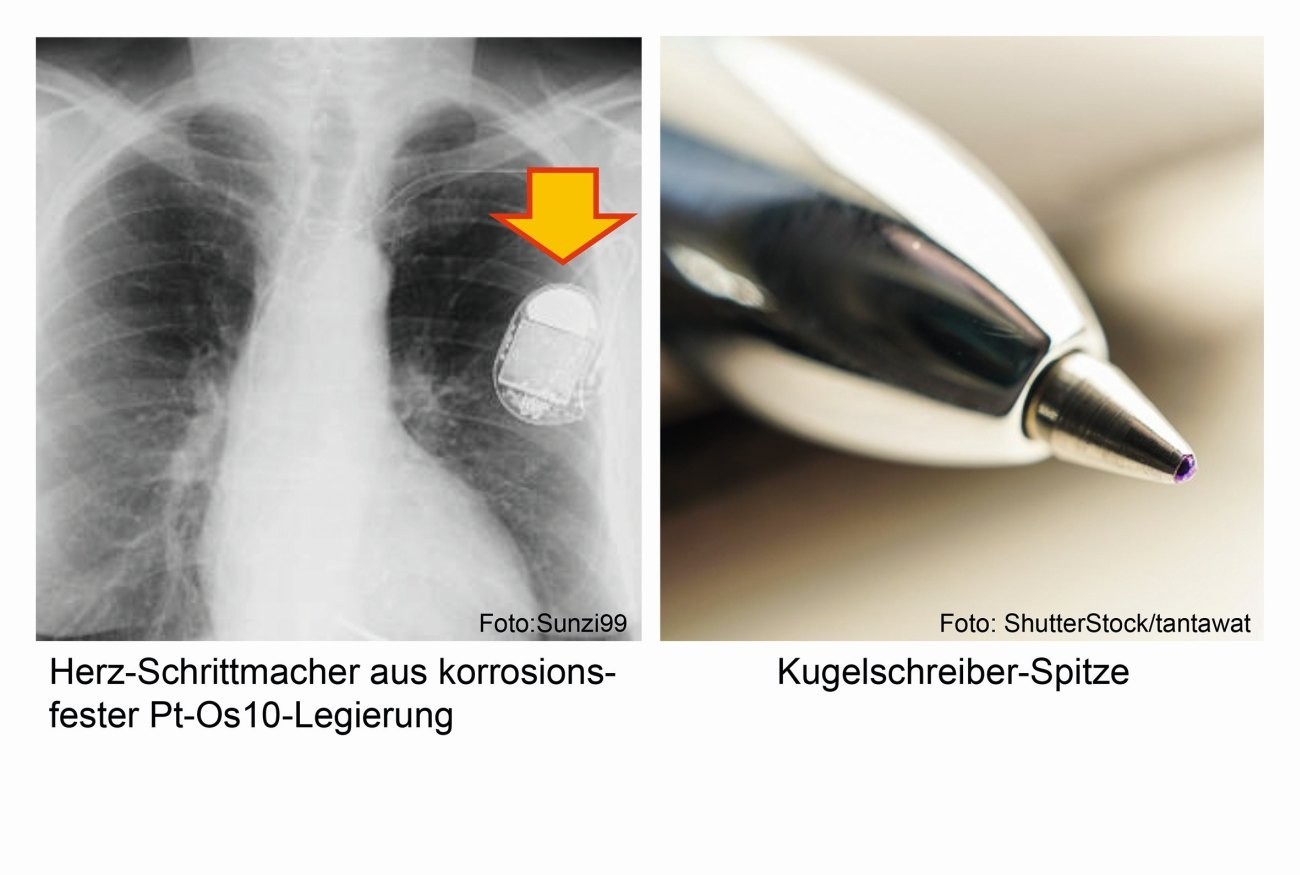 Fig. 9: Examples of applications for osmium alloys
Fig. 9: Examples of applications for osmium alloys
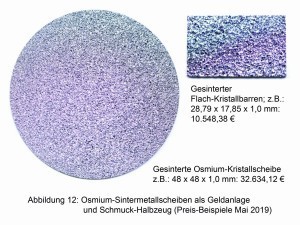
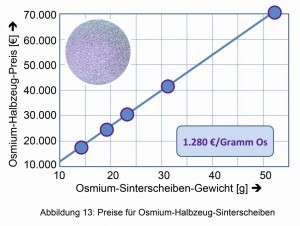
This changed with the production and processing of crystalline osmium metal in 2013 and its marketing as a jewelry and investment metal (Fig. 12) by the newly founded "Osmium Institute" in Baierbrunn [13]. Since the breakthrough in crystallization, the price of one gram of osmium has exploded from just under EUR 13 to over EUR 400. In June 2019, the price of osmium in small quantities was €1,280/gram Os (Fig. 13).
4 Chemical osmium analyses
In addition to modern analytical methods in research and industry, such as atomic absorption (AAS), induced coupled plasma (ICP) or X-ray fluorescence analysis (XRF), classic photometric and quantitative precipitation processes with organic reagents are still used in some cases [14]. As a number of other elements interfere, prior separation as osmium tetroxide, OsO4, is recommended.
As early as 1918, the Moscow chemist Lev Alexandrovich Chugaev (1873-1922) proposed thiourea for the detection of this element. Under defined working conditions, a characteristic red coloration is produced, while the ruthenium produces a blue color.
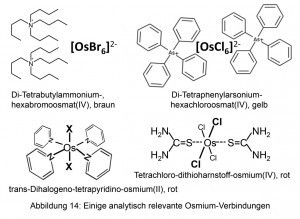
Quaternary alkyl and arylammonium cations as well as the tetraphenylarsonium cation are suitable for the quantitative precipitation of hexahalogenoosmates(IV).
The neutral tetrapyridino-dihalogeno-osmium(II) is also very difficult to dissolve. It is accessible, for example, by boiling the hexahalogenoosmate(IV) in pyridine with glycerol (Fig. 14).
Isotope analyses are somewhat more complex: 186Osis a natural alpha emitter with a half-life of 2.0 x 1015 years. Beta radiation of the rhenium isotope 187Reproduces the stable isotope 187Os. If the geological rhenium content in the deposits is high, the 187Os/186Os ratiocan be used geochronologically [15].
5 Os tetroxide and Os compounds
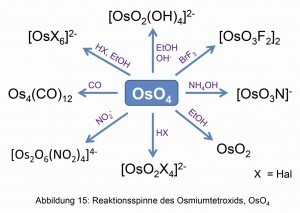
Due to the practical oxidizability of osmium to the tetroxide, OsO4, and the low boiling point, most other osmium compounds start from the osmium tetroxide (Fig. 15).
Osmium(VIII) oxide is produced from the osmium metal by oxidizing osmium solutions with nitric acid or sodium peroxodisulfate in sulfuric acid. Due to its volatility and toxicity, it is sold melted in ampoules [16].
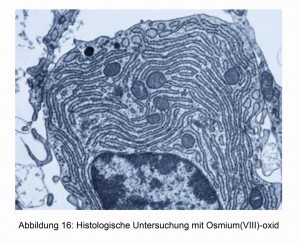
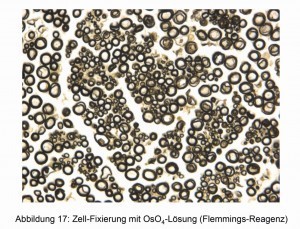
In addition to the applications already described, OsO4 is used in particular for histological examinations [17]. The cell edges take on different colorations and can therefore be easily recognized under the light microscope. Two examples are shown in Figures 16 and 17.
The compound is only slightly soluble in water, but very soluble in carbon tetrachloride, CCl4.
Through photochemical reactions, the halide ions from the halogenated hydrocarbons can attach to the osmium as a ligand [18].
With halogen acids, such as hydrochloric acid, the osmium(VIII) oxide quickly converts to hexahalogeno-osmate(IV) according to the following equation:
OsO4 + 10 HCl → H2[OsCl6] + 2 Cl2 + 4 H2O.
By precipitation from concentrated solutions of potassium hexachloro-osmate(IV), K2OsCl6, with concentrated hydrochloric acid, homogeneous solid solutions are obtained in any desired mixing ratio [19]. The course of the lattice constants follows "Vegard's rule". This rule describes the linear dependence of the lattice constants of a substitutional solid solution or an alloy on the percentage of components [20].
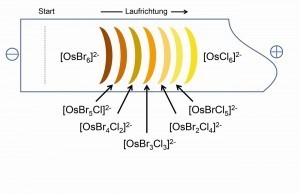
The incomplete conversion of K2[OsCl6] in HBr produces the series of seven mixed ligand complexes of the type K2[OsClxBr6-x], which are separately visible in their typical colors when running to the cathode with the aid of electrophoresis (Fig. 18) and are also accessible by preparation. In this way, an abundance of mixed ligand complexes with various inorganic and organic ligands is accessible [21].
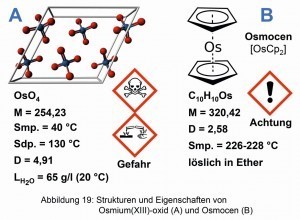
Osmocene is an interesting osmium compound. It was first synthesized by Ernst Otto Fischer (1918-2008) and Heinrich Grumbert, starting from OsO4 [22]. Together with Geoffrey Wilkinson, Fischer was awarded the Nobel Prize in Chemistry in 1973 for his work on organometallic "sandwich compounds" [23]. Applications for photocatalytic water decomposition are said to be quite successful with osmocene [24]. Figure 19 shows the structures and properties of osmium(IV) oxide and osmocene.
Despite the partly thermodynamically and kinetically quite stable osmium preparations, only relatively few compounds are documented with chemical and physical data. This may also be due to the expensive starting materials and the limited applications of this element.
Literature
[1] https://de.wikipedia.org/wiki/Osmium
[2] Wolf, I.: Crystalline osmium, the rarest precious metal on earth, Osmium Institute, presentation at Invest 2019, https://www.insignitus.com/de/blog/kristallines-osmium-das-seltenste-und-dichteste-edelmetall-der-erde/
[3] List of osmium locations at Mineralienatlas and Mindat
[4] Hasenpusch, W.: Dissertation, University of Kiel, 1976
[5] https://www.brandeins.de/magazine/brand-eins-wirtschaftsmagazin/2016/intuition/osmium-os
[6] https://www.chemie-schule.de/KnowHow/Wiedemann-Franzsches_Gesetz
[7] https://www.heraeus.com/en/hch/products_and_solutions_chemicals/chemical_products/pgm_solutions_pc/os_pc/osmium_page_pc.html
[8] https://www.chemicool.com/elements/osmium.html
[9] https://www.azom.com/article.aspx?ArticleID=1842
[10] https://de.wikipedia.org/wiki/Dihydroxylierung
[11] https://www.technology.matthey.com/article/18/3/94-96
[12] Hill, A.F.: Organotransition Metallic Chemistry of Sulfur Dioxide Analogs, Advances in Organometallic Chemistry, 1994
[13] www.osmium-institute.com; https://www.buy-osmium.com/de/barren/disk/
[14] MERCK, Darmstadt: Organic Reagents for Trace Analysis, Osmium, 1975
[15] Faure, G.: Priciples of Isotope Geology, Wiley, N.Y.. (1977), 2nd ed. (1986) 608 pages, RÖMPP Osmium
[16] https://de.wikipedia.org/wiki/Osmium(VIII)-oxide
[17] https://www.chemistryworld.com/podcasts/osmium-tetraoxide/7656.article
[18] Hasenpusch, W.; Preetz, W.: Photochemical ligand exchange on hexahalogenoosmates(IV), ZAAC, 432 (1977) 107-114
[19] Müller, H.; Rödl, M.: The solid solution system K2OsCl6, ZAAC, 608 (1992) 145-146
[20] https://de.wikipedia.org/wiki/Vegardsche_Regel
[21] Preetz, W., Blasius, E.: Gemischtligandkomplexe, I. Allgemeine und theoretische Betrachtungen über Bildung von Gemischtligandkomplexen und ionophoretische Trennmöglichkeiten, 332 (1964) 140-148
[22] https://de.wikipedia.org/wiki/Osmocen
[23] https://de.wikipedia.org/wiki/Ernst_Otto_Fischer
[24] Kunkely, H.; Vogler, A.: Water splitting by light with osmocene as photocatalyst, Angew. Chem, 121(2009) 1713-5