The combination of electroplating and zinc flake coating systems requires careful selection of the zinc and zinc alloys, passivations and topcoat coatings used as well as their process parameters. The systems selected show that the topcoats should be applied no later than 24 hours after the electrolytic zinc alloy coating. Heat treatment for hydrogen de-embrittlement before the topcoat coating leads to an improvement in corrosion protection, especially for zinc-nickel surfaces. The pH range of the passivation used was also much narrower with the given system than it is with a non-post-topcoat coating.
1 Introduction
No influence of the admixtures (aluminum flakes, pigments) on the corrosion process could be determined for the topcoats used. The poly- ester-based topcoats tested exhibited better corrosion protection than the comparable epoxy-based systems. The barrier effect and permeability of a topcoat has a significant influence on corrosion protection. Due to their high barrier effect and low permeability, topcoat coatings can lead to a passive zinc and zinc alloy surface. Corrosion only occurs on damaged areas (pores, cracks, etc.). The presence of a small active and large passive surface leads to pitting corrosion in a chloride-containing environment and to an accelerated attack on the zinc and zinc alloy layer, which leads to localized red rust phenomena. An optimum ratio of passive to active surface (permeability) prevents pitting corrosion and leads to a high level of corrosion protection.
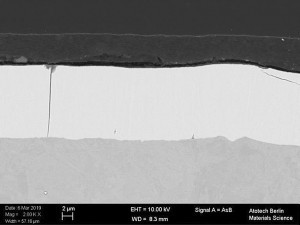
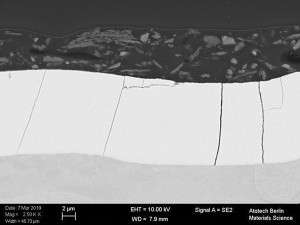
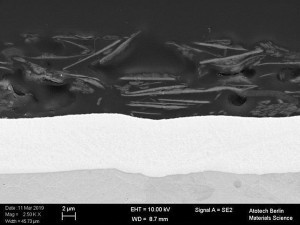
Fig. 1: Cross-section of a transparent (Fig . 1a), black (Fig. 1b) and silver (Fig. 1c) topcoat Fig. 1: Cross-section of a transparent (Fig. 1a), black (Fig. 1b) and silver (Fig. 1c) topcoat on a passivated zinc-nickel surface [3]
Topcoats and sealers are integral components of electroplating systems. Coating takes place in the rack system in baths downstream of the passivation process. In barrel systems, these are usually carried out in separate post-treatment systems in simple heated centrifuges. The topcoats and sealers are dried in this process, and in the rarest cases cross-linking takes place. Their barrier effect leads to a significant increase in corrosion protection, and optical requirements and tribological properties (e.g. coefficients of friction) can also be represented. In recent years, the use of highly complex centrifuges, such as those used in zinc flake coating, has become increasingly common [1]. These centrifuges have a tilting mechanism (tilting) or a so-called "planetary system". This allows significantly more even, but also thicker layers to be applied. The treatment of the topcoats in downstream ovens at temperatures of 180-250 °C enables the use and cross-linking of polyester, epoxy or acrylate resins.
In the further course of the work, only topcoats that were applied in centrifuges for zinc flake coating and crosslinked in downstream ovens are reported on.
In order to achieve a good result, the systems, zinc / zinc alloy, passivation and topcoats must be optimally matched to each other. With regard to the topcoats, the question arises as to which system represents the best combination and how the components of the topcoats and their underlying paint system affect the corrosion mechanism of the entire coating system. For this purpose, various model systems in transparent, silver and black (Fig. 1) based on acrylates, epoxies and polyesters were applied to zinc or zinc alloy coatings. The influence of the pre-treatment (passivated galvanic surface) was also investigated with regard to its corrosion properties.
Electrochemical investigations (impedance spectroscopy and potential measurements) as well as common corrosion measurements (DIN EN ISO 9227 NSST, ACT II according to Volvo Std. VCS 1027,1449) and scanning electron micrographs were used to characterize the topcoat coatings.
2 Experimental
Steel plates (10 x 20 cm) were degreased and then coated with the following systems:
Electrolytic alkaline zinc-nickel (Ni = 12-15 %, 10 µm) + thick film passivation (cobalt free) + spray applied:
a. Polyester-based transparent topcoat
b. Epoxy-based transparent topcoat
c. Polyester-based black topcoat
d. Epoxy-based black topcoat
e. Polyester-based silver topcoat
f. Epoxy-based silver topcoat
Galvanized and zinc-nickel (Ni = 12-15 %w/w) coated (10 µm) + fluoride-free thick-film passivation hexagon head screws (M10-1.5 x 65) were applied in a P&P DS250 centrifuge:
g. Acrylic-based black waterborne topcoat
h. Epoxy-based silver topcoat
All topcoats used are solvent-based (with the exception of topcoat g) and were baked at 210 °C for 30 min after application (with the exception of topcoats a and g at 150 °C).
Electrochemical measurements
The electrochemical measurements were carried out using a PGSTAT302N potentiostat (GPES software) from Metrohm in a measuring cell with a three-electrode arrangement. The working electrode has a measuring area of 10.17 cm2 and a saturated calomel electrode (SCE 0.2412V vs. NHE (25 °C)) was used as a reference electrode. All potentials used in this study were converted to the standard hydrogen electrode. A platinum grid was used as the counter electrode. The measurements were carried out in a so-called neutral salt spray electrolyte (NSST) in accordance with ISO 9227:
50 g/l sodium chloride (p.a.) in DI water (<10 mS/cm), conductivity 70 mS/cm (21 °C), pH 6.9, temp: 35 °C naturally aerated.
The free corrosion potentials (OCP) were measured over a period of one hour, followed by the impedance measurement:
Starting potential: OCP, potential amplitude: 5 mV, frequency range: 100 kHz-10 mHz.
Impedance measurements were carried out at the same measuring point after 1 h, 24 h, 48 h, 72 h, 144 h and 168 h. A repeat measurement was carried out after each measurement time. During the OCP and impedance measurements, the electrolyte temperature was 35 °C; in the intermediate phases it was lowered to RT (22 °C). The impedance measurements were evaluated in the Bode diagram, the total impedance is read at 500 mHz. The sheets were examined with a light microscope before the start of the measurements and after 168 h at the measuring point and the pore density was determined.
Corrosion measurements
Corrosion tests were carried out in the neutral salt spray test (NSST, according to DIN EN ISO 9227) and cyclic tests (ACT II according to Volvo Std. VCS 1027,1449). One sheet in each salt spray test was cross-cut (down to the base material).
Scanning electron microscopy
Topcoated sheets were aged for 0 h, 144 h and 312 h at 22 °C in a neutral salt spray electrolyte. After the aging time, the sheets were rinsed with DI water and dried. Cross-sections were then prepared and measured using SEM/EDX. Measurement areas were drawn in the center of the sheets and the coating thickness was measured magnetically inductively ten times before and ten times after aging.
Topcoat | 0 h µm | 312 µm | Layer increase µm | Remarks |
Polyester transparent | 6,0 | 6,5 | 0,5 | No surface change |
Epoxy transparent | 7,1 | 7,9 | 0,8 | White rust |
Polyester black | 5,7 | 6,5 | 0,8 | Blistering |
Epoxy black | 4,1 | 4,8 | 0,7 | Blistering |
Polyester silver | 5,7 | 5,8 | 0,1 | No surface change |
Epoxy silver | 5,3 | 5,9 | 0,6 | Bubble formation |
3 The various coating systems show clear differences when aged
![Abb. 2: Schema eines Ersatzschaltbildes für ein Metall mit nichtleitender poröser Deckschicht [7] Abb. 2: Schema eines Ersatzschaltbildes für ein Metall mit nichtleitender poröser Deckschicht [7]](/images/stories/Abo-2021-02/thumbnails/thumb_gt-2021-02-0058.jpg) Fig. 2: Schematic of an equivalent circuit diagram for a metal with a non-conductive porous top layer [7]The tests were carried out on selected coating systems (transparent, silver and black). These systems differ significantly from each other (see Fig. 1). Various pigments can be observed in the black topcoat, aluminum flakes in the silver topcoat and the pigment-free paint layer in the transparent topcoat.
Fig. 2: Schematic of an equivalent circuit diagram for a metal with a non-conductive porous top layer [7]The tests were carried out on selected coating systems (transparent, silver and black). These systems differ significantly from each other (see Fig. 1). Various pigments can be observed in the black topcoat, aluminum flakes in the silver topcoat and the pigment-free paint layer in the transparent topcoat.
When the topcoat-coated (topcoats a-f) passivated zinc-nickel sheets were aged for 312 h in a 5 % sodium chloride electrolyte (see Table 1), a clear surface change and increase in coating thickness was observed. With the exception of the polyester-based silver topcoat, all topcoats show a clear increase in coating thickness of 0.5-0.8 µm. With pure water absorption, the increase in coating thickness would be 0.1-0.15 µm [5]. It follows from this that ions have already diffused through the coating even in the sheets without surface changes and corrosion has started at the zinc-nickel-topcoat phase boundary. The fact that, in contrast to corrosion tests in the salt spray cabinet or in cyclic tests, bubble formation is observed is due to the low removal of the corrosion products (including hydroxide ions) when the electrolytes are at rest, as hydroxide ions in particular lead to paint adhesion on the surface [6].
Appropriately treated metal sheets were also used for the impedance measurements. Here too, aging was carried out in a 5 % sodium chloride electrolyte at 22 °C. The measurements were carried out at 35 °C. At the beginning of the measurements and after the last measurement (168 h), the sheets were examined microscopically at the measuring point and the pore density was determined (Table 2). No blistering or white rust formation was observed on any of the sheets at the measuring point.
Topcoat | Pore density / 2.1mm2 | |
Start of ageing | 168 h Ageing | |
Polyester transparent | 1 | 0 |
Epoxy transparent | 30 | 53 |
Polyester black | 78 | 55 |
Epoxy black | 16 | 54 |
Polyester silver | 35 | 24 |
Epoxy silver | 14 | 24 |
It can be seen that the epoxy-based topcoats exhibited an increase in pore density over the ageing period, whereas the polyester-based topcoats showed no increase. At the beginning of each impedance measurement, the free corrosion potential was measured over one hour. Table 3 shows the potentials over the ageing time.
The passivated zinc-nickel coatings in combination with a topcoat all show a more positive OCP (with the exception of polyester silver) than a passivated zinc-nickel coating without a topcoat. The free corrosion potentials are slightly more negative than iron. In the course of ageing, the potentials become slightly more positive or are stable, close to the OCP of iron. Due to the insulating effect of the topcoat, it is not possible to measure the potential in the intact area [5]. The zinc-nickel layer behaves passively in this area. The potentials are set via imperfections and damage. Despite the positive potential, hardly any corrosion is observed. While water diffuses in very quickly, the diffusion of ions (chloride ions) is strongly inhibited, which leads to an extensive diffuse double layer at the zinc-nickel-topcoat phase boundary. This is considered to be the main cause of the low corrosion rates [5].
Coating | OCP /VSHE | ||
1 h ageing | 72 h Removal from storage | 168 h ageing | |
Iron | -0,43* | ||
Polyester transparent | -0,52 | -0,45 | -0,44 |
Epoxy transparent | -0,49 | -0,35 | -0,36 |
Polyester black | -0,46 | -0,48 | -0,51 |
Epoxy black | -0,47 | -0,49 | -0,44 |
Polyester silver | -0,69 | -0,47 | -0,49 |
Epoxy silver | -0,48 | -0,48 | -0,47 |
4 The ratio of barrier effect / permeability of a topcoat has a major influence on corrosion protection
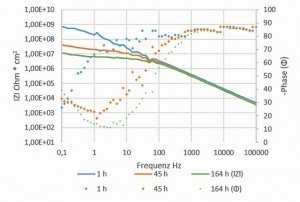 Fig. 3: Bode diagram after three ageing times in 5 % NaCl solution at 35 °C. Layer structure: Alk. Zinc-nickel (Ni: 14 %w/w) + Co-free thick-film passivation + epoxy-based transparent topcoat Impedance measurements were carried out to investigate the influence of the barrier effect and permeability of a topcoat. Figure 2 shows the equivalent circuit diagram for a metal with a non-conductive porous topcoat [7]. The measured impedances consist of the electrolyte resistance, the resistance in the pores and impurities as well as the topcoat and the polarization resistance. There is a cover layer capacitance parallel to the cover layer resistance and a double layer capacitance parallel to the polarization resistance. The electrolyte resistance is negligible in the overall system. It was not possible to differentiate between the resistance in the pores, the top layer and the polarization resistance.
Fig. 3: Bode diagram after three ageing times in 5 % NaCl solution at 35 °C. Layer structure: Alk. Zinc-nickel (Ni: 14 %w/w) + Co-free thick-film passivation + epoxy-based transparent topcoat Impedance measurements were carried out to investigate the influence of the barrier effect and permeability of a topcoat. Figure 2 shows the equivalent circuit diagram for a metal with a non-conductive porous topcoat [7]. The measured impedances consist of the electrolyte resistance, the resistance in the pores and impurities as well as the topcoat and the polarization resistance. There is a cover layer capacitance parallel to the cover layer resistance and a double layer capacitance parallel to the polarization resistance. The electrolyte resistance is negligible in the overall system. It was not possible to differentiate between the resistance in the pores, the top layer and the polarization resistance.
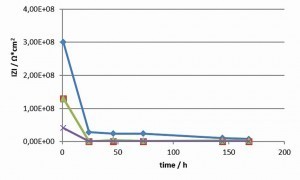
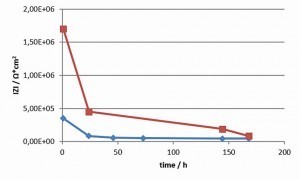
The total impedance was plotted against the frequency in a Bode diagram. The impedance at 500 mHz was read off as the value for the individual topcoat systems. Figure 3 shows an example of a Bode diagram (epoxy-based transparent topcoat) at three different ageing times. Each impedance measurement was immediately followed by a repeat measurement. The results were almost identical, the system is less reactive and is almost in equilibrium (Tab. 4).
Coating | IZIOhm*cm2 | ISO 9227 NSST | ACTII FordL467 | |||
1 h | 168 h | RR/h | RR in % in Ritz after 2232 h | Cycl. to RR | RR in the scribe | |
Polyester transparent | 0,35*106 | 0,045*106 | >2000 | 0 | >15 | >15 |
Epoxy transparent | 300*106 | 8,0*106 | 2000 | 100 | >15 | 8 |
Polyester black | 130*106 | 0,5*106 | >2000 | 0 | 11 | 2 |
Epoxy black | 130*106 | 2,9*106 | >2000 | 0 | >15 | 5 |
Polyester silver | 34*106 | 0,6*106 | >2000 | 0 | >15 | 5 |
Epoxy silver | 1,7*106 | 0,085*106 | >2000 | 10 | >15 | 3 |
Irrespective of the applied system, the total impedances drop significantly within the first 24 h and then remain at a low level or drop slightly (see Figs. 4 and 5). As the drop in the first 24 h is independent of the pore density and is also observed in the low-porosity polyester-based transparent topcoat, it can be assumed that the conductivity of the coating system increases. In contrast to the work of S. Palraj et al [2], an influence of the components of the individual topcoats, transparent, silver or black (pigments, aluminum flakes) on the diffusion speed of the ions could no longer be observed in this time frame. However, it must be taken into account that the tested coating systems were optimized with regard to their corrosion protection. In comparable coating systems (alkyd resin coatings), Stratmann et al [5] describe that saturation of the coating with water occurs within 10 minutes. However, as saturation with water does not lead to a significant increase in conductivity, only the incorporation of chloride ions can lead to a corresponding increase in conductivity in the selected system. The measurements suggest that a stable state of saturation with ions has been reached after 24 hours. The epoxy-based systems still show a moderate further drop after 24 h to 168 h, whereas this is significantly lower in the polyester-based systems. This is consistent with the observed change in the pore density of the two coating systems. The pore density of the epoxy-based coatings increased significantly, whereas it remained constant in the polyester-based systems. Jags in the Bode diagram at medium and low frequencies are due to pitting.
If one compares the total impedances of the individual systems with the corrosion results in the salt spray test and ACT II test (Tab. 4, Fig. 6 and 7), paying particular attention to the areas with a cross-section, it can be seen that all systems exhibit a high level of corrosion protection.
The topcoat with the highest permeability (lowest resistance) shows the best results in the corrosion test with regard to cathodic corrosion protection. Even after 2232 h in the salt spray test and 15 cycles in the ACT II test, the polyester-based transparent topcoat still shows no red rust (not even in the cross-cut) (see Fig. 6).
Fig. 6: Coating with the highest permeability Fig. 6: Coating with the highest permeability (lowest total impedance), polyester transparent 2232 h (DIN EN ISO 9227 NSST), 15 cycles (ACT II according to Ford L467)
In general, a total impedance of 45,000 Ohm * cm2 can be rated as high in terms of its barrier effect [4]. The corrosion phenomena observed are therefore due to disturbances (pores, cracks) and the storage of water and ions in the paint layer caused by the precipitation in the sodium chloride electrolyte. The fact that the paint with the highest permeability (lowest total impedance) has the best corrosion properties is due to a larger active surface. Coatings with a low permeability have a large passive surface, corrosion only occurs at the damaged areas (comparable to pitting corrosion) and cathodic corrosion protection is reduced [5]. The optimum ratio of barrier effect and permeability leads to maximum corrosion protection. The early formation of red rust [8] occasionally observed with the zinc-nickel, black passivation and topcoat coating system is not exclusively due to a shift in the free corrosion potential OCP of this system towards values of the OCP for iron or more positive, but also to the passive zinc-nickel surface (caused by the insulating topcoat layer) in the case of isolated defects.
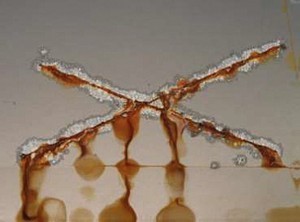
Fig: 7: Coating with the lowest permeability Fig: 7: Coating with the lowest permeability (highest total impedance), epoxy transparent2232 h (DIN EN ISO 9227 NSST), 15 cycles (ACT II according to Ford L467)
5 The topcoat should be applied no later than 24 h after the galvanic zinc-nickel coating, heat aging increases the corrosion protection
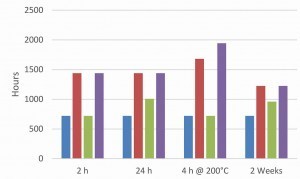
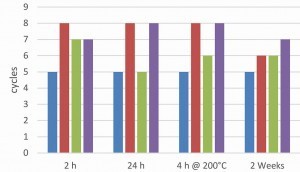
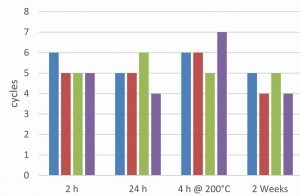
In order to investigate the influence of the pre-treatment on the overall system after the topcoat application, passivated, electrolytically coated screws were aged for different lengths of time or subjected to hydrogen de-embrittlement. A water-based or solvent-based topcoat was then applied once or twice. The aim was to investigate whether the ageing of the passivated surface has an influence on the overall system. Regardless of the ageing conditions and the topcoats used, all coatings showed excellent adhesion in the tape and cross-cut test. Figures 8 and 9 show the salt spray test results of the two coating systems on a galvanized and zinc-nickel coated surface. A fluoride-free thick-film passivation at pH 2.2 was used as passivation.
In the case of the zinc-nickel surfaces, a topcoat applied two weeks later showed a significant drop in corrosion protection. This applies to both paint systems. This phenomenon does not occur with the zinc surface. The zinc-nickel surface shows that heat treatment to remove hydrogen embrittlement increases corrosion protection, which is not the case with a zinc surface. Such an observation has already been discussed in the literature [8]. In general, the zinc-nickel surface shows significantly higher corrosion protection for both topcoats. The ACT II tests confirm the results of the salt spray test for the epoxy-based silver topcoat (Fig. 10).
However, the positive influence of the zinc-nickel coating is much less pronounced. Again, it can be seen that a heat treatment for hydrogen de-embrittlement before applying the topcoat has no negative influence on the corrosion protection. With the acrylate-based black topcoat (Fig. 11), the ACT II results show no real differences between a zinc and zinc-nickel base coat. However, the best results are again achieved after heat treatment. In most cases, a second topcoat does not show any significant increase in corrosion protection. This is due to coating in the laboratory centrifuge under low mechanical stress. On an industrial scale, a second topcoat layer would be useful to compensate for any damage to the first layer.
6 The passivation used must be applied in a significantly narrower pH range for subsequent topcoating
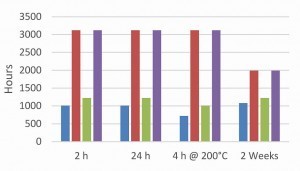
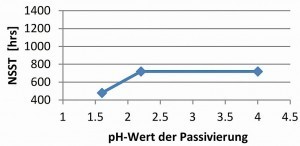
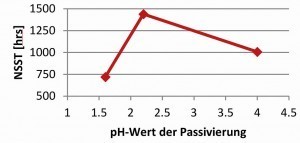
With the same fluoride-free thick-film passivation on zinc and zinc-nickel surfaces, an influence of the pH value of the passivation on the corrosion protection of the overall system was observed (Fig. 12 and 13).
At pH values of pH 1.6, a significant drop in corrosion protection is observed. This cannot be attributed to a change in surface roughness, as this did not change when the pH value was varied with the given passivation [3]. Adhesion problems were also not observed [3]. The pH value has a strong influence on the layer thickness and composition of the passivation [8]. At low pH values, the passivation layer re-dissolves. The extent to which the layer thickness of the passivation has an influence on the behavior of the topcoat must be determined by further tests. In general, it can be seen that with increasingly complex systems, the selection of the individual layers, zinc or zinc-nickel, passivation including bath parameters and the selected topcoats must be closely coordinated. If, for example, passivation on zinc-nickel in the range of pH 1.6-2.5 can lead to uniform corrosion results, this is not always the case in combination with a topcoat as shown in the example.
7 Summary
The combination of electroplating and zinc flake coating systems enables surface finishers to meet the highest demands of the automotive industry. Corrosion protection results can be achieved (>2000 h to red rust) that go far beyond the typical standards (1000 h to red rust). The systems used must be closely coordinated. Zinc-nickel surfaces in particular should be coated with topcoat within 24 hours. Heat treatment for hydrogen de-embrittlement prior to topcoating leads to an improvement in corrosion protection, especially for zinc-nickel surfaces. The pH range of the passivation used was much narrower with the given topcoat coatings than with a coating without topcoat. The thickness and composition of the passivation has an influence on the adhesion of the topcoat and the corrosion protection of the overall system. No influence of the admixtures (pigments, aluminum flakes) on the corrosion process could be determined for the topcoats used. The polyester-based topcoats used exhibited better corrosion protection than the corresponding epoxy-based systems. When aged in an aqueous sodium chloride solution, the epoxy-based topcoats show an increase in pores, which could not be observed with the polyester-based topcoats. In the case of screw coatings in the laboratory centrifuge, most tests with one and two topcoats did not show any major difference in corrosion protection. This is due to the low mechanical stress in the laboratory centrifuge. In practice, the mechanical stress would be much higher, so that a second coating makes sense.
Topcoat coatings have a high barrier effect and low permeability. This results in a passive zinc or zinc alloy surface, whereby corrosion only occurs in damaged areas (pores, cracks, etc.). With small active and large passive surfaces, pitting corrosion occurs in chloride-containing electrolytes. This results in accelerated attack on the zinc and zinc alloy surface and can lead to localized red rust. An optimum ratio of passive to active surface (permeability) prevents pitting corrosion and leads to a high level of corrosion protection.
Literature
[1] P. Hülser: Electroplating technology, 10 (2018), 1964
[2] S. Palraj: Progress in Organic Coatings, 67 (2010), 399
[3] V. Levermann: Master Thesis, Investigation of Corrosion Properties of Electrolytic Zinc and Zinc Alloys in Combination with Zinc Flake Coatings, Fachhochschule Südwestfalen, 2019
[4] P. Hülser: Galvanotechnik, 9 (2014), 1872
[5] M. Stratmann et al.: color + varnish 100, 2 (1994), 93
[6] J. Sander et al.: Korrosionsschutz durch Beschichtungen, Hanover, Vincentz Network, 2011, farbe + lack Edition
[7] Knoblauch: womag issue (2015) 9/31
[8] S. Sengl; P. Hülser: Galvanotechnik 4 (2018) 655