This article presents the results of a current literature search and our own laboratory tests from an R&D project to develop processes for the electrodeposition of palladium and its alloys from aprotic ionic liquids. The evaluation of literature data and our own laboratory tests show that, in principle, a number of technically interesting palladium alloys such as Pd/Ag, Pd/Au, Pd/Sn, Pd/In, Pd/Cu and Pd/Ni can be deposited from air and moisture-stable ionic liquids of the type [Bmim]+ [BF4/Cl]-. The composition of the individual alloy layers can be directly influenced by the initial concentrations of the individual metal species and by the current form (galvanostatic vs. pulse current). At present, the aprotic deposition of Pd alloy layers with a layer thickness of > 1 µm is still complex. On the other hand, a large number of different Pd alloys can be electrochemically deposited from the ionic liquids presented.
1 Introduction
![Abb. 1: Pd-Anteil in den Pd/Ag-Schichten in Abhängigkeit vom Potential (aus [16], Grafik vereinfacht) Abb. 1: Pd-Anteil in den Pd/Ag-Schichten in Abhängigkeit vom Potential (aus [16], Grafik vereinfacht)](/images/stories/Abo-2021-01/thumbnails/thumb_gt-2021-01-0005.jpg) Fig. 1: Pd content in the Pd/Ag layers as a function of potential (from [16], simplified graphic)In recent years, interest in the deposition of palladium alloys has increased continuously. Palladium and its alloys are important coating materials for a wide range of technical applications due to their wear resistance, corrosion resistance, electrical conductivity or catalytic activity. Palladium-silver alloys are used for hydrogen sensors [1-4], as a storage medium for hydrogen produced from renewable energy [5], or as a catalytically active material for oxidation reactions of organic compounds (e.g. methanol) [6, 7]. In low-current applications, palladium alloys (e.g. palladium-silver, palladium-nickel) are used as electrical contact surfaces, whereby palladium contents of between 30 % and 50 % are common for applications on relay contacts for the palladium-silver coatings [8]. Bimetallic palladium-platinum catalysts are known for their high activity for methane combustion, although they are subject to phase transformation and structural changes [9-11]. Nanocrystallites of palladium-platinum are reported to have increased CO tolerance compared to pure platinum crystallites, which can result in increased stability in the electrochemical oxidation of formic acid [12].
Fig. 1: Pd content in the Pd/Ag layers as a function of potential (from [16], simplified graphic)In recent years, interest in the deposition of palladium alloys has increased continuously. Palladium and its alloys are important coating materials for a wide range of technical applications due to their wear resistance, corrosion resistance, electrical conductivity or catalytic activity. Palladium-silver alloys are used for hydrogen sensors [1-4], as a storage medium for hydrogen produced from renewable energy [5], or as a catalytically active material for oxidation reactions of organic compounds (e.g. methanol) [6, 7]. In low-current applications, palladium alloys (e.g. palladium-silver, palladium-nickel) are used as electrical contact surfaces, whereby palladium contents of between 30 % and 50 % are common for applications on relay contacts for the palladium-silver coatings [8]. Bimetallic palladium-platinum catalysts are known for their high activity for methane combustion, although they are subject to phase transformation and structural changes [9-11]. Nanocrystallites of palladium-platinum are reported to have increased CO tolerance compared to pure platinum crystallites, which can result in increased stability in the electrochemical oxidation of formic acid [12].
The main problems with the electrodeposition of palladium alloys from conventional aqueous electrolytes are the considerable difference in the deposition potentials for the palladium and its alloying metal (e.g. indium), the sometimes low hydrogen overvoltage and the high solubility of hydrogen in metallic palladium or its alloys. Efforts are therefore being made to minimize these electroplating problems. A new technical approach could be electrochemical deposition from non-aqueous or aprotic electrolytes such as ionic liquids. Compared to molecular (organic) solvents, ionic liquids have the advantage that they are non-volatile (very low vapor pressure), are thermally stable over a wider temperature range (sometimes > 150 °C) and have a comparatively large electrochemical potential window (at electrolyte temperatures between 30-100 °C sometimes in the range of -2 V to +1 V vs. common reference electrodes for ionic liquids such as Fc/Fc+, Al/Al3+ or Pd/Pd2+). Various air- and moisture-stable ionic liquids are now commercially available on the market for research and development work. For the electrodeposition of metals and alloys, it is necessary to dissolve the corresponding metals in the selected ionic liquids in sufficient concentration. Such a dissolution process is possible, for example, by adding halide ions (e.g. chloride ions) in excess, so that stable soluble metal complex ions (e.g. [PdCl4]2-) are formed.
To date, there are only a handful of publications in the specialist literature that deal with the topic of electrochemical deposition of palladium alloys from ionic liquids. This article is therefore intended to summarize the current status of R&D projects on the electrochemical deposition of palladium alloys from ionic liquids as an overview article.
2 Palladium alloys
2.1 Palladium-silver
![Abb. 2: Pd-Anteil in den Pd/Ag-Schichten, abgeschieden aus [Bmim][BF4/Cl] (f = 0,33 Hz, t = 60 min) Abb. 2: Pd-Anteil in den Pd/Ag-Schichten, abgeschieden aus [Bmim][BF4/Cl] (f = 0,33 Hz, t = 60 min)](/images/stories/Abo-2021-01/thumbnails/thumb_gt-2021-01-0014.jpg) Fig. 2: Pd content in the Pd/Ag layers, deposited from [Bmim][BF4/Cl] (f = 0.33 Hz, t = 60 min)The physical and chemical properties of silver as a contact material are well described in the literature [13, 14]. Disadvantages of pure silver coatings are the tendency to tarnish, the low hardness, the tendency to cold welding and the tendency to material migration [8]. This has led to the development of silver alloy materials such as silver-nickel or silver-copper with increased hardness. Alloys with aluminum, palladium or zinc exhibit significantly better tarnish resistance than silver [13]. Palladium-silver (with approx. 40 % Pd) shows good tarnish resistance in a sulphurous atmosphere as well as sufficient resistance to material migration [8]. The state diagram of palladium-silver shows complete solid solution formation [8, 15]. In principle, this indicates that from a thermodynamic point of view, any palladium-silver mixing ratio can be set in the deposited layer. In principle, it should also be possible to produce the desired alloy compositions by electrodeposition.
Fig. 2: Pd content in the Pd/Ag layers, deposited from [Bmim][BF4/Cl] (f = 0.33 Hz, t = 60 min)The physical and chemical properties of silver as a contact material are well described in the literature [13, 14]. Disadvantages of pure silver coatings are the tendency to tarnish, the low hardness, the tendency to cold welding and the tendency to material migration [8]. This has led to the development of silver alloy materials such as silver-nickel or silver-copper with increased hardness. Alloys with aluminum, palladium or zinc exhibit significantly better tarnish resistance than silver [13]. Palladium-silver (with approx. 40 % Pd) shows good tarnish resistance in a sulphurous atmosphere as well as sufficient resistance to material migration [8]. The state diagram of palladium-silver shows complete solid solution formation [8, 15]. In principle, this indicates that from a thermodynamic point of view, any palladium-silver mixing ratio can be set in the deposited layer. In principle, it should also be possible to produce the desired alloy compositions by electrodeposition.
The aprotic deposition of palladium-silver alloys from a Lewis-based ionic liquid of the type 1-ethyl-3-methylimidazolium tetrafluoroborate chloride ([Emim][BF4/Cl]) is described by a group of authors led by I.-W. Sun [16]. The electrochemical deposition was carried out here potentiostatically using constant deposition potentials in the range from -0.53 V to -0.73 V (vs. Al/Al3+) at a bath temperature of 35 °C. The Pd content in the alloys increased with increasingly negative potentials and began to level off at a constant level of -0.65 V when the reduction of Ag(I) and Pd(II) reached its mass transfer limiting range. As the mass fraction of Pd(II)/Ag(I) in the electrolyte increased, the Pd fraction in the alloy deposits increased. However, the mass fraction of Pd/Ag in the precipitates was lower than in the electrolyte because the diffusion coefficient of the Pd(II) complex ions was lower than that of the Ag(I) species. When the deposition was diffusion controlled, the composition of the alloys was less dependent on the concentration of Pd(II) and Ag(I) as long as the Pd(II)/Ag(I) concentration ratio was constant. An increase in temperature caused an increase in the metal deposition rate, as the viscosity of the ionic liquid decreased and the mass transfer of the dissolved metal ions was accelerated.
Investigations using an X-ray diffractometer (XRD) showed that homogeneous Pd/Ag alloy layers were obtained in the temperature range from 35 °C to 120 °C, which suggests that Pd and Ag are soluble in each other under these boundary conditions and that homogeneous solid solution formation took place. The composition of the produced Pd/Ag precipitates was mainly determined by the ratio Pd(II)/Ag(I) concentration in the electrolyte and the diffusion coefficients of the two metal species. The surface morphology of the produced Pd/Ag alloy deposits was predominantly spherical-granular and compact [16].
Figure 1 shows the determined Pd content in the deposited Pd/Ag layers as a function of the given deposition potential.
In our own experiments, the electrodeposition of palladium-silver from an ionic liquid of the type 1-butyl-3-methylimidazolium-tetrafluoroborate-chloride ([Bmim][BF4/Cl]) with a molar ratio of the anions tetrafluoroborate [BF4-]/choride [Cl-] of 0.9:0.1 was carried out as part of the BMBF joint project GALACTIF. This (less expensive) ionic liquid can be easily prepared by mixing [Bmim][BF4] with [Bmim][Cl]. Investigations using cyclic voltammetry (CV) showed that in the ionic liquid [Bmim][BF4/Cl], which has comparable physico-chemical properties to [Emim][BF4/Cl], the Ag ions are already reduced a few millivolts before the Pd species, i.e. that the deposition potential of the two metals is also very close to each other here and alloy deposition is also possible here. For the deposition of palladium-silver, the metal salts AgCl and PdCl2 were dissolved in [Bmim][BF4/Cl]. In contrast to the authors Sun et al., the experiments on the fem were not carried out in a glove box with an inert gas atmosphere but in a normal air atmosphere, as the base electrolyte itself is stable in air and moisture. Round copper rods (as rotating rod electrodes) with a diameter of 4 mm and a surface area of 2.6 cm2 were used as substrates. However, deposition was carried out here using pulsed current deposition (ton/toff = 1s/2s, relative duty cycle/duty cycle q = 50 %, pulse frequency f = 0.33 Hz) and a bath temperature of T = 80 °C. With increasing (average pulse) current density jm (from 0.05 to 0.3 mA/cm2), the amount of palladium in the Pd/Ag precipitates increased, whereby the limiting current density was already reached at a current density of 0.3 mA/cm2 with low bath agitation (Fig. 2).Photomicrographs using a scanning electron microscope (SEM) show that fine crystalline, compact and spherical surface structures (see Fig. 3) with very few pores were preferentially obtained by means of pulse current deposition, which exhibited comparatively good adhesion to the copper substrates. At current densities in the range of 0.1 to 0.3 mA/cm2, Pd/Ag layer thicknesses of 0.3 to 0.6 µm could be produced at a bath temperature of 80 °C and a deposition time of 60 min.
Fig. 3: SEM images of Pd/Ag layers deposited by pulse current from [Bmim][BF4/Cl];
Bath temperature = 80 °C, metal content = 23.5 mmol Pd(II) + 25.5 mmol Ag(I);
Left image: average pulse current density jm = 0.1 mA/cm2; center image: jm = 0.2 mA/cm2; right image: jm = 0.3 mA/cm2
2.2 Palladium-gold
Galvanostatic deposition of palladium-gold alloys from the Lewis-based ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate chloride is also reported by the group of authors led by I.-W. Sun [17]. Potentiostatic deposition experiments resulted in precipitates that adhered poorly to the selected substrate (Ni foil). Galvanostatic depositions from the ionic liquid, on the other hand, yielded Pd/Au coatings that adhered well to the Ni substrate. Electrochemical deposition was carried out at bath temperatures of 30 °C, 80 °C and 120 °C at current densities between 0.3 and 3 mA/cm2. No homogeneous Pd/Au alloy layers were obtained at low electrolyte temperatures. This indicates that Au and Pd were deposited "individually". However, a deposition temperature of 120 °C increased the interdiffusion between Pd and Au and thus enabled the formation of homogeneous Pd/Au alloy layers [17]. The deposition experiments also showed that the Pd content in the deposited alloy layers increased with increasing current density (from 0.4 mA/cm2 to approx. 1 mA/cm2) until the applied current density reached the mass transfer limiting value for the reduction of Pd(II) complex ions (→ limiting current density). When the applied current density (e.g. 0.4 mA/cm2) was lower than the sum of the individual limiting current densities of Pd(II) and Au(I), the partial current due to Pd(II) decreased with increasing temperature due to the increasing partial current of Au(I). As a result, the Pd content in the alloy layers decreased with increasing electrolyte temperature. On the other hand, when the applied current density was high enough so that the deposition of both Au and Pd was limited within the selected temperature range, the temperature did not significantly affect the composition of the alloy deposits. For the deposits in the limiting current density range, the Pd content in the Pd/Au alloys is slightly lower than the Pd(II) mass fraction in the electrolyte, since the diffusion coefficient of the Pd(II) complex ions is smaller than that of the Au(I) species [17].
X-ray diffraction (XRD) studies of the deposits showed that homogeneous Pd/Au alloy layers were obtained in the temperature range from 80 °C to 120 °C, suggesting that Pd and Au are soluble in each other under these boundary conditions and that homogeneous solid solution formation occurred during deposition. In general, the surface morphology of the Pd/Au deposits was granular and compact, with the size of the granular crystallites decreasing with increasing current density while the Pd content increased [17]. Figure 4 above shows the calculated Pd content in the deposited Pd/Au alloy layers.
2.3 Palladium-tin
Some palladium alloys are technically interesting materials primarily because of their excellent catalytic properties. For example, the electrochemical oxidation of ethanol is important for various chemical-technical applications. Compared to Pt/Ru electrodes, Pt/Sn-coated graphite electrodes are considered to be more active for the electrochemical oxidation of ethanol [18]. However, the high price of platinum limited its application in the past. Pd/Sn could represent an alternative, although the current price of palladium is unlikely to bring any direct economic advantages. While applications have been found for Pd/Sn alloys in the removal of nitrate with hydrogen [19], the catalytic coupling of ethyne and ethene [20], for CO oxidation [21] and the electrochemical oxidation of formic acid [22], there are very few studies on the oxidation of ethanol in alkaline solutions [23]. Therefore, in the study by I.-W. Sun et al., the deposition of palladium-tin alloys from the ionic liquid [Emim][BF4/Cl] was carried out and, in addition, initial investigation results on the possible suitability of Pd/Sn deposits produced electrochemically from ionic liquid for the electrochemical oxidation of ethanol were presented [23]. For the electrodeposition of Pd/Sn alloys, SnCl2 anhydride and PdCl2 anhydride were dissolved in [Emim][BF4/Cl]. Tin(II) chloride was chosen because tin(IV) chloride is highly hygroscopic and volatile. The authors used either indium tin oxide-coated glass electrodes (ITO) or graphite electrodes as substrates [23].
A comparison of the cyclic voltamograms (CV curves) of solutions with Sn(II) and Sn(IV) showed that during the cathodic forward run the redox couple Sn(IV)/Sn(II) is reduced in a potential range close to that of Sn(II)/Sn(0). However, during the anodic reflow in the CV, the oxidation potential of Sn(II) to Sn(IV) was much more positive compared to the Sn(0)/Sn(II) redox couple. The large gap between the reduction and oxidation peaks also indicates that the Sn(IV)/Sn(II) redox pair represents an irreversible charge transfer process. In the monometallic electrolyte solutions, the metal deposition of tin took place at a more negative potential compared to palladium. In the various investigations using cyclic voltammetry, the authors also found that Sn(II) ions were chemically oxidized to Sn(IV) by Pd(II) ions in the electrolyte solution according to the following reaction equation [23]:
Sn2+ + Pd2+ → Sn4+ + Pd0 Eq. <1>
For the electrodeposition experiments, however, only Sn(IV)-containing electrolyte solutions were used, which were obtained by oxidizing SnCl2 solutions, among other things. If dissolved Sn(II) was oxidized to Sn(IV) in advance, the [SnCl6]2 species formed were stable in the ionic liquid and could thus be used for PdSn alloy deposition. Pd/Sn deposition was performed potentiostatically at deposition potentials in the range of -0.40 V to -1.10 V (vs. Al/Al3+). At a deposition potential around -0.40 V, the tin content in the precipitates is low and increases at more negative potentials until a leveling off to a constant value occurred at about -0.80 V (vs. Al/Al3+). Palladium requires a lower overpotential than Sn and metal deposition is mass transfer-limited for both metals at potentials more negative than -0.80 V. Pd-rich palladium-tin alloys were deposited from the electrolyte with higher Pd content (30 mmol Pd(II) + 20 mmol Sn(IV)) and Sn-rich alloys were deposited from the electrolyte with higher Sn(IV) content (20 mmol Pd(II) + 30 mmol Sn(IV)) [23]. Figure 5 shows the calculated Pd content in the Pd/Sn layers as a function of the specified deposition potential for two different electrolyte solutions.
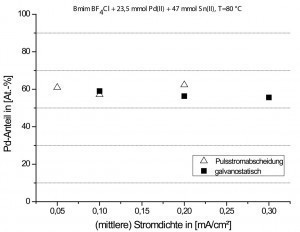 Fig. 6: Pd content in the deposited Pd/Sn layers as a function of the current density For metal deposition on ITO substrate, Pd/Sn solid solutions with fcc crystal structure were obtained for deposits with a Pd content of more than 84 at.%, for deposits up to a Pd content of 62 at.% the intermetallic phase Pd3Sn2 was obtained. For Pd contents of up to 56 at.% in the precipitates, the intermetallic phase Pd/Sn was detected in the investigations using an X-ray diffractometer (XRD). The precipitates deposited from the electrolyte with a higher Pd content were essentially spherical, compact and with good adhesion to the ITO substrates. Homogeneous and comparatively smooth precipitates were obtained at a deposition potential of -0.60 V (vs. Al/Al3+) and a bath temperature of 120 °C. When the Sn content in the alloys was increased (electrolyte with 20 mmol Pd(II) + 30 mmol Sn(IV)), increasingly discontinuous, polyhedral (multi-faceted) surface structures were obtained in the deposits on the ITO substrates instead of a spherical, compact and smooth surface morphology [23].
Fig. 6: Pd content in the deposited Pd/Sn layers as a function of the current density For metal deposition on ITO substrate, Pd/Sn solid solutions with fcc crystal structure were obtained for deposits with a Pd content of more than 84 at.%, for deposits up to a Pd content of 62 at.% the intermetallic phase Pd3Sn2 was obtained. For Pd contents of up to 56 at.% in the precipitates, the intermetallic phase Pd/Sn was detected in the investigations using an X-ray diffractometer (XRD). The precipitates deposited from the electrolyte with a higher Pd content were essentially spherical, compact and with good adhesion to the ITO substrates. Homogeneous and comparatively smooth precipitates were obtained at a deposition potential of -0.60 V (vs. Al/Al3+) and a bath temperature of 120 °C. When the Sn content in the alloys was increased (electrolyte with 20 mmol Pd(II) + 30 mmol Sn(IV)), increasingly discontinuous, polyhedral (multi-faceted) surface structures were obtained in the deposits on the ITO substrates instead of a spherical, compact and smooth surface morphology [23].
The electrodeposition of palladium-tin from the Lewis-based ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate chloride ([Bmim][BF4/Cl]) was also tested as part of the GALACTIF joint project. Investigations using cyclic voltammetry showed that the Sn(II) ions were reduced in the ionic liquid [Bmim][BF4/Cl] at a significantly more negative potential compared to the Pd(II) species. Round copper rods with a diameter of 4 mm and a surface area of 2.6 cm2 were used as substrates. Deposition experiments were carried out both galvanostatically (j = 0.1-0.3 mA/cm2) and by means of pulse current deposition (ton/toff = 1s/2s, load cycle = 50 %, pulse frequency = 0.33 Hz) at a bath temperature of 80 °C. From an electrolyte with a metal content of 23.5 mmol Pd(II) + 23.5 mmol Sn(II), only metallic Pd layers with a very low tin content (< 5 at.-%) could be deposited at a current density between 0.1 and 0.3 mA/cm2. In contrast, layers with a tin content of up to 40-45 at.% could be deposited from an electrolyte with twice the tin content (47 mmol) and a constant Pd concentration. In the Pd/Sn electrolyte with a higher Sn content, the side reaction of the oxidation of Sn(II) to Sn(IV) described by Sun et al [29] could also be observed. This Pd/Sn electrolyte turned increasingly black with increasing electrolysis time due to the formation of colloidal Pd nanoparticles. With current densities in the range of 0.05 to 0.3 mA/cm², Pd/Sn layer thicknesses of 0.2 to 0.3 µm could be deposited with this electrolyte system at a bath temperature of 80 °C and a deposition time of 60 min. Figure 6 shows the calculated Pd content in the deposited alloy layers as a function of the current density from the electrolyte.
The Pd/Sn layers deposited using pulse current were more homogeneous and compact overall compared to the layers that were deposited galvanostatically. In the current density range from 0.05 to 0.3 mA/cm2, spherical surface structures were preferentially obtained, with isolated pores and cracks appearing in the deposited Pd/Sn layers. Figure 7 shows an example of the surface morphology of various Pd/Sn layers.
Fig. 7: SEM images of Pd/Sn layers deposited by means of pulse current from [Bmim][BF4/Cl];
Bath temperature = 80 °C; metal content = 23.5 mmol Pd(II) + 47 mmol Sn(II);
Left picture: Average pulse current density jm = 0.05 mA/cm2; middle image: jm = 0.1 mA/cm2; right image: jm = 0.2 mA/cm2
2.4 Palladium-indium
![Abb. 8: Pd-Anteil in Pd/In-Schichten in Abhängigkeit vom Abscheidepotential ([30], Grafik vereinfacht) Abb. 8: Pd-Anteil in Pd/In-Schichten in Abhängigkeit vom Abscheidepotential ([30], Grafik vereinfacht)](/images/stories/Abo-2021-01/thumbnails/thumb_gt-2021-01-0008.jpg) Fig. 8: Pd content in Pd/In coatings as a function of deposition potential ([30], graphic simplified) Palladium-indium alloys are also of technical interest, as the incorporation of indium into palladium improves microhardness and wear resistance, among other things, without impairing the low contact resistance of palladium. Pd/In coatings can replace pure gold, silver and palladium coatings in various applications. There are already several examples of Pd/In deposition from aqueous electrolytes in the literature [24], while there are hardly any published results for electrodeposition from ionic liquids.
Fig. 8: Pd content in Pd/In coatings as a function of deposition potential ([30], graphic simplified) Palladium-indium alloys are also of technical interest, as the incorporation of indium into palladium improves microhardness and wear resistance, among other things, without impairing the low contact resistance of palladium. Pd/In coatings can replace pure gold, silver and palladium coatings in various applications. There are already several examples of Pd/In deposition from aqueous electrolytes in the literature [24], while there are hardly any published results for electrodeposition from ionic liquids.
The deposition of palladium-indium alloys from the Lewis-based ionic liquid 1-ethyl-3- methylimidazolium tetrafluoroborate chloride was also investigated by the working group of I.-W. Sun and the first fundamental results were presented [24]. For the electrochemical deposition of Pd/In alloys, InCl3 anhydrite and PdCl2 anhydrite were dissolved in the starting liquid [Emim][BF4/Cl]. Nickel foil (A = 0.5 x 0.5 cm2) was used as the substrate. For the Lewis-based ionic liquid (excess of free chloride ions), it is assumed that Pd(II) and In(III) are present as [PdCl4]2- and [InCl5]2- complex anions in the anhydrous ionic liquid, respectively. A comparison of CV curves of solutions with Pd(II) and In(III) showed that the deposition potential of indium is significantly more negative than that of palladium. The considerable difference between the individual deposition potentials indicates that electrochemical deposition of compact Pd/In layers is generally difficult. In the CV of the alloy electrolyte, the deposition potentials of Pd(II) and In(III) shift to more negative potential values compared to the electrolyte solutions with the individual metal species. In the anodic potential scan, the dissolution peak for indium decreased significantly and a new anodic peak appeared in the CV. The changes in the anodic region of the CVs are interpreted by the authors as an indication of the formation of different Pd/In alloy phases. In addition, an underpotential deposition (UPD) of indium on palladium was deduced from the CV data. The Pd/In depositions were carried out potentiostatically by the authors at an electrolyte temperature of 120 °C on Ni substrate. In general, the indium content in the precipitates increased slightly when the deposition potential changed from -0.3 to -0.7 V (vs. Al/Al3+), which corresponds to the UPD range of indium on palladium. At potentials more negative than -0.7 V (OPD of indium), the In content in the samples increased significantly until the deposition range was reached in which the indium deposition in mass transfer was limited [24].
The surface morphology of the precipitates, which is more positive than -0.7 V in a potential range - the range of simultaneous deposition of Pd and underpotential deposition (UPD) of indium - essentially consists of uniformly distributed spherical structures. At significantly more negative deposition potentials - the region of the simultaneous deposition of Pd and the in-volume phase (OPD) - increasingly dendritic surface structures are formed, which may indicate that the deposits do not consist of a homogeneous alloy, but of a mixture of Pd, In and intermetallic phases of Pd/In [24]. Figure 8 shows the determined Pd content in the Pd/In alloy layers produced as a function of the deposition potential.
2.5 Palladium-copper
![Abb. 9: Pd-Anteil in PdCu-Schichten in Abhängigkeit vom Abscheidepotential ([28], Grafik vereinfacht) Abb. 9: Pd-Anteil in PdCu-Schichten in Abhängigkeit vom Abscheidepotential ([28], Grafik vereinfacht)](/images/stories/Abo-2021-01/thumbnails/thumb_gt-2021-01-0009.jpg) Fig. 9: Pd content in PdCu layers as a function of the deposition potential ([28], simplified graphic) Palladium-copper alloys are of technical interest for the separation and storage of hydrogen [25], the catalytic decomposition of nitrate and nitrite [26] and for the oxidation of carbon monoxide. Studies have shown that the incorporation of Cu into Pd increases the electron transfer rate and can therefore improve the catalytic efficiency [27]. Various techniques such as sputtering, electroless deposition and electrochemical deposition are available for the production of Pd/Cu layers. The electrochemical deposition of Pd/Cu from aqueous electrolytes is also impaired here by the low hydrogen overvoltage and the high solubility of hydrogen in the metallic Pd/PdCu layers.
Fig. 9: Pd content in PdCu layers as a function of the deposition potential ([28], simplified graphic) Palladium-copper alloys are of technical interest for the separation and storage of hydrogen [25], the catalytic decomposition of nitrate and nitrite [26] and for the oxidation of carbon monoxide. Studies have shown that the incorporation of Cu into Pd increases the electron transfer rate and can therefore improve the catalytic efficiency [27]. Various techniques such as sputtering, electroless deposition and electrochemical deposition are available for the production of Pd/Cu layers. The electrochemical deposition of Pd/Cu from aqueous electrolytes is also impaired here by the low hydrogen overvoltage and the high solubility of hydrogen in the metallic Pd/PdCu layers.
The electrodeposition of palladium-copper from a Lewis-based ionic liquid of the type [Emim][BF4/Cl] was also investigated by the group of authors led by I.-W. Sun [28]. In addition, initial test results were presented on the effectiveness of the Pd/Cu precipitates produced with regard to a possible application for the electrochemical oxidation of ethanol. For the deposition of palladium-copper, CuCl2 anhydride and PdCl2 anhydride were dissolved in [Emim][BF4/Cl]. Indium tin oxide (ITO) coated glass substrates with an area of 0.5 x 0.5 cm2 were used as substrates. Various CV studies on electrolyte solutions with constant Pd content but different contents of Cu(II) in the solution indicate that underpotential deposition (UPD) of copper on ITO substrate occurs when the Cu content in the electrolyte is lower than that of Pd. At higher copper concentrations, the UPD of Cu(II) is suppressed, which therefore presumably only takes place if Pd has been deposited previously and sufficiently. There is also some evidence that the deposition of Pd in the presence of Cu(II) species is kinetically inhibited [28].
The deposition of Pd/Cu was carried out potentiostatically at a bath temperature of 120 °C on ITO substrate. At deposition potentials of -0.35 to -0.60 V (vs. Al/Al3+), which corresponds to the deposition range of palladium and the UPD range of copper on palladium, Pd/Cu precipitates with a uniform, spherical surface structure were deposited. At potentials more negative than -0.60 V (OPD of copper), the surface morphology of the precipitates was less uniform and compact. Figure 9 shows the amount of Pd in the produced Pd/Cu layers as a function of the given deposition potential.
2.6 Palladium-nickel
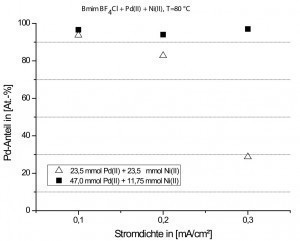 Fig. 10: Pd content in the Pd/Ni layers as a function of the current density and the electrolyte compositionPalladium-nickel alloy layerscan replace hard gold layers in low-current contacts [29]. Nanoporous Pd/Ni alloys are also successfully used for the production of electrochemical sensors. Pd/Ni alloys with magnetic properties can also be deposited as multilayers and can therefore be used for a wide range of technical applications.
Fig. 10: Pd content in the Pd/Ni layers as a function of the current density and the electrolyte compositionPalladium-nickel alloy layerscan replace hard gold layers in low-current contacts [29]. Nanoporous Pd/Ni alloys are also successfully used for the production of electrochemical sensors. Pd/Ni alloys with magnetic properties can also be deposited as multilayers and can therefore be used for a wide range of technical applications.
The aprotic deposition of Pd/Ni from ionic liquids was also investigated as part of the above-mentioned joint project. The deposition experiments were carried out in the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate chloride. A characterization of the electrolyte system using cyclic voltammetry (CV) revealed that the deposition of Ni(II) ions took place at significantly more negative potentials compared to the Pd(II) ions. Since the deposition potentials of Ni(II) and Pd(II) are comparatively far apart, the electrochemical deposition of Pd/Ni is also more difficult here. NiCl2 and PdCl2 were dissolved in [Bmim][BF4/Cl] for the deposition experiments. Round copper rods were again used as substrates. The deposition was carried out both galvanostatically and by means of pulse current deposition (ton/toff = 1s/2s, load cycle = 50 %, pulse frequency = 0.33 Hz) at a bath temperature of 80 °C for two different Pd/Ni electrolyte solutions. In the ionic liquid with 23.5 mmol Pd(II) + 23.5 mmol Ni(II), the Ni content in the Pd/Ni layers increased with increasing current density. In contrast, only metallic Pd layers with a very low Ni content (< 4 %) could be deposited from the electrolyte with 47 mmol Pd(II) + 11.75 mmol Ni(II) (see Fig. 10). With pulse current deposition, the limiting current density is already reached from an (average pulse) current density of 0.3 mA/cm2. As a result, it was no longer possible to deposit well adhering Pd/Ni layers.
The surface images of various Pd/Ni layers obtained using SEM show that spherical surface structures were preferentially deposited here, which were less compact and rougher compared to the Pd/Ag and Pd/Sn layers investigated. The possible influence of the metal content in the electrolyte and the type of current (constant direct current vs. pulsed current) on the surface morphology of the Pd/Ni layers produced is illustrated by the SEM images in Figure 11.
In the current density range of 0.1 to 0.3 mA/cm2, Pd/Ni layers with layer thicknesses of 0.05 to 0.2 µm were deposited at a bath temperature of 80 °C and a deposition time of 60 min.
Fig. 11: Surface morphology of Pd/Ni layers, deposited from [Bmim][BF4/Cl]; bath temperature = 80 °C;
Left image: galvanostatic, j = 0.2 mA/cm2, metal content = 47 mmol Pd(II) + 11.75 mmol Ni(II);
Image center: galvanostatic, j = 0.2 mA/cm2, cMe = 23.5 mmol Pd(II) + 23.5 mmol/l Ni(II);
Right image: pulsed current deposition, with ton/toff = 1s/2s, jm = 0.2 mA/cm2, cMe = 23.5 mmol Pd(II) + 23.5 mmol Ni(II)
3 Summary
The results presented here from a recent literature review and an ongoing BMBF project on the electrodeposition of palladium alloys from ionic liquids show that a number of technically interesting palladium alloys (Pd/Ag, Pd/Au, Pd/Cu, Pd/In and Pd/Ni) can be produced from air- and moisture-stable ionic liquids of the type [Emim]+[BF4/Cl]- or [Bmim]+[BF4/Cl]-. [Bmim]+[BF4/Cl]- can be easily deposited on common substrate materials such as copper, graphite or ITO.
In the temperature range of 50-120 °C, spherical-granular surface structures are preferably obtained for the metallic deposits. The composition of the deposited alloy layers can be directly influenced to a certain extent by the initial content of the individual metal species in the alloy electrolyte, by the current form (galvanostatic vs. pulse current) and variation of the current density/deposition potential.
Electrolytes based on ionic liquids generally have a significantly higher viscosity and lower conductivity compared to aqueous electrolytes. As a result, the mass transfer of metal ions in these electrolyte systems is significantly lower, which means that lower metal deposition rates are achieved, as often only comparatively low current densities can be used for metal deposition. However, higher electrolyte temperatures (80-120 °C) and increased electrolyte flow can significantly improve mass transfer in these novel electrolyte systems.
According to the current state of knowledge, which is based on the results of very little R&D work to date, the electrodeposition of palladium alloy layers with a layer thickness of > 1 µm from ionic liquids is comparatively complex. In contrast, compared to aqueous electrolytes, the deposition of a larger variety of different palladium alloys appears to be possible. This could be of technical interest for the production of special electrodes with catalytic coating or for the electrodeposition of thin films, for example.
Acknowledgments
The results presented were obtained as part of the sub-project "Hydrogen-free deposition of precious metals and precious metal alloy layers from ionic liquids" (project no. 13XP5017B) in the joint project "New electroplating coating processes from ionic liquids" (GALACTIF). The authors would like to take this opportunity to thank the Federal Ministry of Education and Research (BMBF) for the financial support of the project and Dr. Fellenberg from the VDI-TZ project management organization for the excellent project support.
THE AUTHORS
Dr. Reinhard Böck
Dr. Mila Manolova
Research Institute Precious Metals & Metal Chemistry (fem), Department of Electrochemistry - Electroplating - Corrosion, Schwäbisch Gmünd
Literature
[1] B. Sharma; J.S. Kim: Graphene decorated Pd-Ag nanoparticles for H2sensing, Int. J. Hydrogen Energy 43, 2018, 11397-11402
[2] J.S. Jang; S. Qiao; S.-J. Choi; (...) I.-D. Kim; R.M. Penner: Hollow Pd-Ag composite nanowires for fast responding and transparent hydrogen sensors, ACS Appl. Mater. Interfaces 9, 2017, 39464-39474
[3] L. Tang; L. Yu; G. Si; W. Ou; Y. Qiao: Pd-Ag alloy dendritic nanowires. Fabrication and application in hydrogen sensor, J. Nanosci. Nanotechnol. 11, 2011, 10255-10261
[4] E. Yue; G. Yu; Y. Ouyang; B. Weng; W. Si; L. Ye: Electrochemical fabrication of Pd-Ag alloy nanowire arrays in anodic alumina oxide template, J. Mater. Sci. 24, 2008, 850-856
[5] F. Fang; C. Zhao; G. Zhang; Z. Zheng; J. Ding; Q. Shao; L. Geng: Morphology engineering of Au/Pd/Ag alloy nanostructures for enhanced electrocatalytic ethanol oxidation, Part. Part. Syst. Charact. 35, 2018, 78-89
[6] H. Mori; K. Sano; T. Kobayashi; H. Yamashita: Surface engineering of a supported PdAg catalyst for hydrogenation of CO2 to formic acid: Elucidating the active Pd atoms in alloy nanoparticles, J. Am. Chem. Soc. 140, 2018, 8902-8909
[7] N. Benipal; J. Qi; Q. Liu; W. Li: Carbon nanotube supported PdAg nanoparticles for electrocatalytic oxidation of glycerol in anion exchange membrane fuel cells, Appl. Chem. Catal. B Environ. 210, 2017, 121-130
[8] F. Talgner; U. Manz; S. Berger; B. Weymüller; A. Pfund: New silver-palladium electrolyte for electrical contacts, Jahrbuch Oberflächentechnik, Eugen G. Leuze Verlag, Vol. 71, 2015, 37-42
[9] H. Nassiri; R.E. Hayes; N. Semagina: Stability of Pd-Pt catalysts in low-temperature wet methane combustion: Metal ratio and particle reconstruction, Chem. Eng. Sci. 186, 2018, 44-51
[10] H. Geng; L. Zhang; Z. Yang; Y. Yan; J. Ran: Effect of Pd/Pt ratio on the reactivity of methane catalytic combustion in bimetallic Pd-Pt catalyst, Int. J. Hydrogen Energy 43, 2018, 11069-11078
[11] W.S. Wilburn; M.S. Epling: A summary of sulfur deactivation, desorption, and regeneration characteristics of mono- and bimetallic Pd-Pt methane oxidation catalysts: Pd:Pt mole ratio and particle size dependency, Emiss. Control Sci. Technol. 4, 2018, 78-89
[12] Y. Li; F. Hao; Y. Wang; Y. Zhang; C. Ge; T. Lu: Facile synthesis of octahedral Pt-Pd nanoparticles stabilized by silsesquioxane for the electrooxidation of formic acid, Electrochim. Acta 133, 2014, 302-307
[13] E. Vinaricky (ed.): Electrical contacts, their materials and applications, Springer Verlag, 2002, 201
[14] B. Gehlert: Contact behavior of thin galvanic PdAg/AuCo layers as contact materials for connectors in comparison to AuCo layers of the same thickness, 44th VDA-Fachber., VDE-Verlag, 1993
[15] ULLMAN'S Encyclopedia of Industrial Chemistry, Wiley & Sons, 8th Edition, Release 2013
[16] C.-C. Tai; F.-Y. Su; I.-W. Sun: Electrodeposition of palladium-silver in a lewis basic 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate ionic liquid, Electrochim. Acta 50, 2005, 5504-5509
[17] F.-Y. Su; J.-F. Huang; I.-W. Sun: Galvanostatic deposition of palladium-gold alloys in a lewis basic EMI-Cl-BF4 ionic liquid, J. Electrochem. Soc. 151, 12, 2004, C811-C815
[1]8 W. Zhou; Z. Zhou; S. Song; W. Li; G. Sun; P. Tsiakaras; Q. Xin: Pt based anode catalysts for direct ethanol fuel cells, Appl. Catal. B 46, 2, 2003, 273-285
[19] H. Berndt; L. Mönnich; B. Lücke; M. Menzel: Tin promoted palladium catalysts for nitrate removal from drinking water, Appl. Catal. B 30, 1-2, 2001, 111-122
[20] J.M. Hill; J. Sheu; R.M. Watwe; A. Dumesic: Microcalorimetric infrared spectroscopic and DFT studies of ethylene adsorption on Pd and Pd/Sn catalysts, Langmuir 16, 2000, 2213-2219
[21] J. Araña; P. Ramirez de la Piscina; J. Liorca; J. Sales; N. Homs; J.L.G. Fierro: Bimettalic silica-supported catylsts based on Ni-Sn, Pd-Sn, and Pt-Sn as materials in the CO oxidation reaction, Chem. Mater. 10, 5, 1998, 1333-1342
[22] Z. Liu; X. Zhang: Carbon supported PdSn nanoparticles as catalysts for formic acid oxidation, Electrochem. Commun. 11, 8, 2009, 1667-1670
[23] L.-H. Jou; J.-K. Chang; T.-J. Whang; I.-W. Sun: Electrodeposition of Palladium-Tin Alloys from 1-Ethyl-3-methylimidazolium Chloride-Tetrafluoroborate Ionic Liquid for Ethanol Electro-Oxidation, J. Electrochem. Soc. 157, 8, 2010, D443-D449
[24] S.-I Hsiu; C.-C. Tai; I.-W. Sun: Electrodeposition of palladium-indium from 1-ethyl-3-methylimidazolium chloride tetrafluoroborate ionic liquid, Electrochimica Acta, 51, 2006, 2607-2613
[25] J.B. Miller; B.D. Morreale; A.J. Gellman: The effect of adsorbed sulfur on surface segregation in a polycrystalline Pd70Cu30 alloy, Surf. Sci. 602, 10, 2008, 1819-1825
[26] O.M. Ilinitch; F.P. Cuperus; V. Nosova; E.N. Gribov: Catalytic membrane in reduction of aqueous nitrates: operational principles and catalytic performance, Catal. Today 56, 1-2, 2000, 137-145
[27] M. Fernandez-Garcia; A. Martinez-Arias; C. Belver; J.A. Anderson; J.C. Conesa; J. Soria: Behavior of palladium-copper catalysts for CO and NO elimination, J. Catal. 190, 2000, 387- 395
[28] L.-S. Jou; J.-K. Chang; T.-J. Twhang; I.-W. Sun: Electrodeposition of palladium-copper films from 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate ionic liquid on indium tin oxide electrodes, J. Electrochem. Soc. 156, 6, 2009, D193-D197
[29] F. Simon: Palladium-nickel instead of gold, Galvanotechnik, 53, 1999, 30-33




![Abb. 4: Pd-Anteil in den Pd/Au-Schichten in Abhängigkeit der Stromdichte ([17], Grafik vereinfacht) Abb. 4: Pd-Anteil in den Pd/Au-Schichten in Abhängigkeit der Stromdichte ([17], Grafik vereinfacht)](/images/stories/Abo-2021-01/thumbnails/thumb_gt-2021-01-0018.jpg)
![Abb. 5: Pd-Anteil in den Pd/Sn-Schichten in Abhängigkeit vom Potential (aus [29], Grafik vereinfacht) Abb. 5: Pd-Anteil in den Pd/Sn-Schichten in Abhängigkeit vom Potential (aus [29], Grafik vereinfacht)](/images/stories/Abo-2021-01/thumbnails/thumb_gt-2021-01-0019.jpg)






