5.7 Palladium preparations
Palladium usually has an oxidation number of +2 in its compounds. In complexes and chelates, the doubly positively charged palladium occurs as the central atom of square planar compounds, as is also known from copper, nickel and platinum. The Pd(II) compounds can easily be brought into the tetravalent oxidation state by oxidation, which, however, reverts to divalent palladium at elevated temperatures:
[PdCl4]2- + Cl2 ↔ [PdCl6]2-
The hexachloridopalladate(IV), which is relatively poorly soluble as a potassium or ammonium salt, decomposes at 60 °C back into the readily soluble tetrachloridopalladate(II).
In addition to the more common oxidation states +2 and +4, palladium is also found less frequently in oxidation states 0 and +5. The most popular palladium compounds and their known properties are listed in Table 2.
|
Name |
Formula |
Color |
M |
Smp. [°C] |
D (20 °C) |
LH2O (20 °C) [g/l] |
Signal word |
H-phrases |
|
Pd(II) oxide |
PdO |
black |
122,42 |
750 (Z.) |
8,3 |
poorly soluble |
Danger |
272 |
|
Pd(IV).oxide |
PdO2 |
black |
138,42 |
200 (Z.) |
poorly soluble |
Attention |
272-413 |
|
|
Pd(II) sulphide |
PdS |
brown |
138,49 |
950 (Z.) |
6,7 |
poorly soluble |
Attention |
315-319-335 |
|
Pd(II) fluoride |
PdF2 |
light violet |
144.42 |
952 |
5,76 |
Hydrolysis |
||
|
Pd(II) chloride |
PdCl2 |
reddish brown |
177,3 |
590 (subl.) |
4,0 |
poorly soluble |
Danger |
290-302-317-318-410 |
|
Pd(II)-bromide |
PdBr2 |
black |
266,2 |
310 (Z.) |
5,35 |
poorly soluble |
Attention |
315-319-335 |
|
Pd(II) iodide |
PdI2 |
black |
360,2 |
350 (Z.) |
6,0 |
slightly soluble |
Attention |
317 |
|
Pd(II)-cyanide |
Pd(CN)2 |
yellow |
158,5 |
26 |
2,2 |
poorly soluble |
Danger |
330-310-300-400-410 |
|
Pd(II) tetramine chloride |
[Pd(NH3)4]Cl2 |
light yellow |
245,4 |
well soluble |
||||
|
Pd(II) tetramine hydrogen carbonate |
[Pd(NH3)4] (HCO3)2 |
light yellow |
264,4 |
169-174 |
2,04 |
soluble |
hazardous |
317-318-372-410 |
|
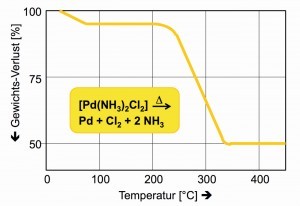
Diamminedichlorido- Pd(II) |
[Pd(NH3)2Cl2] |
yellow |
211,4 |
2,75 |
||||
|
Diammindinitro- Pd(II) |
[Pd(NH3)2(NO2)2] |
light yellow |
232,5 |
poorly soluble |
||||
|
Pd(II) nitrate |
Pd(NO3)2 - 2 H2O |
reddish brown |
266,7 |
Hydrolysis |
Danger |
272-315-319-335 |
||
|
Pd(II) sulphate |
PdSO4 |
reddish brown |
202,5 |
Hydrolysis |
Danger |
314 |
||
|
Pd(II) acetate |
Pd(CH3COO)2 |
golden brown |
224,5 |
205 (Z.) |
2,19 |
poorly soluble |
danger |
317-318-319 |
|
Ammonium hexachloridopalladate (IV) |
(NH4)2[PdCl6] |
dark red |
355.2 |
Attention |
302-315-319 |
The density of the palladium(II) cyanide was interpolated from the densities of analogous compounds (Fig. 14).
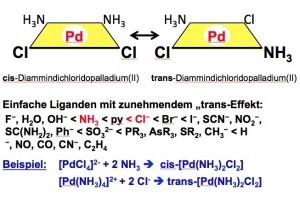 Fig. 15: Selective isomer synthesis using the "trans effect" Since palladium powders are usually also produced from the chloride in the preparation plants, manufacturers have numerous special requirements and specifications. This is because electrically deposited powders have a different habitus than chemically precipitated powders with formaldehyde, hydrazine, boranate or many other reducing agents under various parameter conditions. Many palladium salts are based on the knowledge gained from experiments with platinum compounds.
Fig. 15: Selective isomer synthesis using the "trans effect" Since palladium powders are usually also produced from the chloride in the preparation plants, manufacturers have numerous special requirements and specifications. This is because electrically deposited powders have a different habitus than chemically precipitated powders with formaldehyde, hydrazine, boranate or many other reducing agents under various parameter conditions. Many palladium salts are based on the knowledge gained from experiments with platinum compounds.
For example, the Danish chemist Sophus Mads Jørgensen (1837-1914), son of a master tailor, was able to show that the "Vauquelin salt", which was the first palladium complex presented by the French pharmacist and chemist Louis Nicolas Vauquelin (1763-1829), is to be understood as a compound analogous to the green platinum salt of the Berlin physicist and chemist Heinrich Gustav Magnus (1802-1870), the "Magnus salt" [37]. The flesh-red "Vauquelin's salt" [Pd(NH3)4] [PdCl4] is analogous to the green "Magnus salt" [Pt(NH3)4] [PtCl4]. As a cation-anion compound in a 1:1 ratio, these double complexes are also relatively poorly soluble in water. Neutral complexes are generally just as poorly soluble in water.
According to the work of the French chemist J. Reiset (1818-1896), who developed the compounds tetramminpalladium(II) chloride, (Reiset salt I), [Pt(NH3)4]Cl2 and trans-diamminedichloridoplatin(II), (Reiset salt II), [Pt(NH3)2Cl2] and the isomeric cis-diamminedichloroplatin(II), (Peyrone salt) of the Italian chemist Michele Peyrone (1813-1883), the corresponding palladium(II) complexes have also been prepared.
For the stereoselective production of isomeric, planar cis and trans complexes, the Russian chemist Ilya Ilyich Chernyaev (1893-1966) discovered the "trans effect" in 1926 [38, 39].
If, for example, tetrachloridopalladate(II) is used as a starting point, the monoammine complex is obtained by reaction with ammonia in the first step. Due to the stronger trans-directing properties of the chlorido ligand compared to the ammine ligand, a second ammonia molecule is incorporated in the trans position to a chlorido ligand and thus in the cis position to the first ammine ligand (Fig. 15).
The zero oxidation state of palladium is found, for example, in bis(tri-tert-butylphosphine)palladium(0) (Fig. 16), which is used as a catalyst and whose structure was determined by Japanese scientists in 1992 [40].
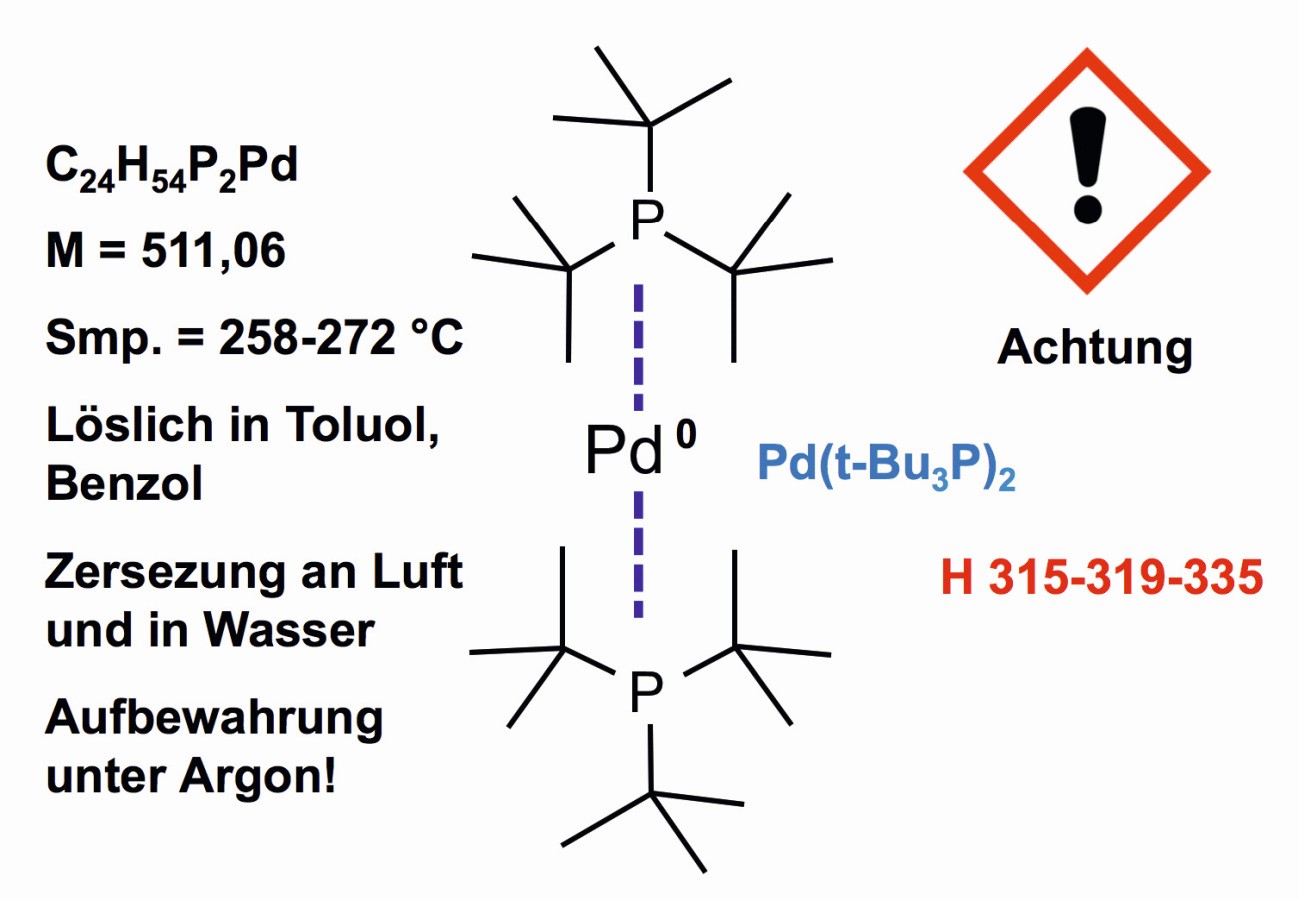 Fig. 16: Palladium in valence state zero in bis(tri-tert-butylphosphine)palladium(0)
Fig. 16: Palladium in valence state zero in bis(tri-tert-butylphosphine)palladium(0)
The compound is available on the precious metal preparation market. It is produced under inert gas. The compound plays a role among the new generation of catalysts for organic syntheses [41].
5.8 Galvanic baths
 Fig. 17: Explosive palladium compoundsThevery good corrosion and temperature resistance also makes palladium interesting for use as a protective coating for base materials.
Fig. 17: Explosive palladium compoundsThevery good corrosion and temperature resistance also makes palladium interesting for use as a protective coating for base materials.
Weakly alkaline electrolytes from the series of light platinum metals with palladium produce a color similar to ruthenium or rhodium. High-gloss, white and highly decorative surface layers down to 0.5 µm can be achieved with these baths.
Palladium baths are well suited for creating barrier layers. They are used when the final layer is to be rhodium, for example, as these two precious metals are very similar in color [42].
The jewelry and eyewear industry uses palladium baths with 2 g palladium/liter for high-gloss, decorative and bright palladium layers up to 0.5 µm thick.
Precious metal alloys can also be electroplated. For example, a silver-palladium 10 alloy is very suitable for connector contacts that are used in high-pole or high-temperature applications. The electroplating process allows stable process control with consistent layer properties. The coating hardness, the contact resistance and the tribological properties of the alloy are stable over longer periods of up to 200 °C. The vibration resistance of the contact layer is also given with contacting to standard silver [43].
Efforts are often made to use halogen-free electrolytes for electroplating baths. A range of nitrates and palladium compounds with amine and nitrite ligands are used for this purpose (Fig. 17). When handling and processing these compounds, it should be noted that they can react explosively [44].
6 Palladium analyses
In the analytical laboratories of precious metal companies, the physical determination methods of X-ray fluorescence analysis and the multi-element determination method ICP (Induce Coupled Plasma) dominate. Nevertheless, classic precipitation with dimethylglyoxime is also used as a reference analysis for palladium [45].
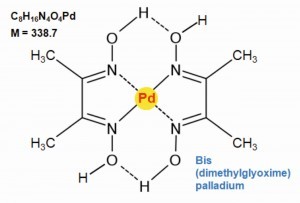
Dimethylglyoxime is used to precipitate palladium(II) quantitatively from solutions of dilute mineral acids at pH = 2 as bis(dimethylglyoximato)-palladium(II) (Fig. 18). In a basic environment, at a pH value above 9, the precipitate dissolves again.
The dimethylglyoxime-nickel and platinum complexes have the same composition as the palladium compound, but they dissolve in acids and thus allow the quantitative separation of palladium from nickel and platinum [46, 47].
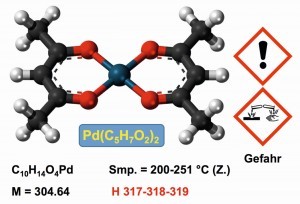
Alternative poorly soluble palladium compounds are the neutral complexes with acetylacetonate (Fig. 19). The compound also serves as a catalyst [48]. With fluorinated acetylacetonate ligands, metal coatings can even be carried out in a vacuum by thermal decomposition.
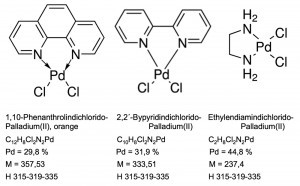
Even diamminedichloridopalladium(II), [Pd(NH3)2Cl2], is relatively insoluble in water at room temperature. If the ammonia ligands are replaced by organic diamine compounds such as ethylenediaminedichlorido-palladium or 2,2'-bypyridinedichlorido-palladium as well as 1,10-phenanthrolinedichlorido-palladium, they can also be used analytically in the context of quantitative precipitation operations (Fig. 20).
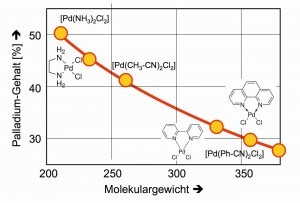
If the palladium contents are weighed against the molecular weights of the poorly soluble complexes in a diagram, it can be seen how the size of the ligands can improve the results of the palladium analyses because weighing errors have a correspondingly smaller effect (Fig. 21).
A very interesting chemistry involves the porphyrin-palladium derivatives [49], which enable quantitative determinations due to their lack of solubility in water, alcohol, ether and alkanes, as the palladium content in these compounds is particularly low (Fig. 22). The solubility of these neutral complexes in acetonitrile, dichloromethane or dimethylformamide (DMF), as well as in small quantities in acetone, also enables quantitative photometric determinations of these red complexes, which also serve as robust photocatalysts [50].
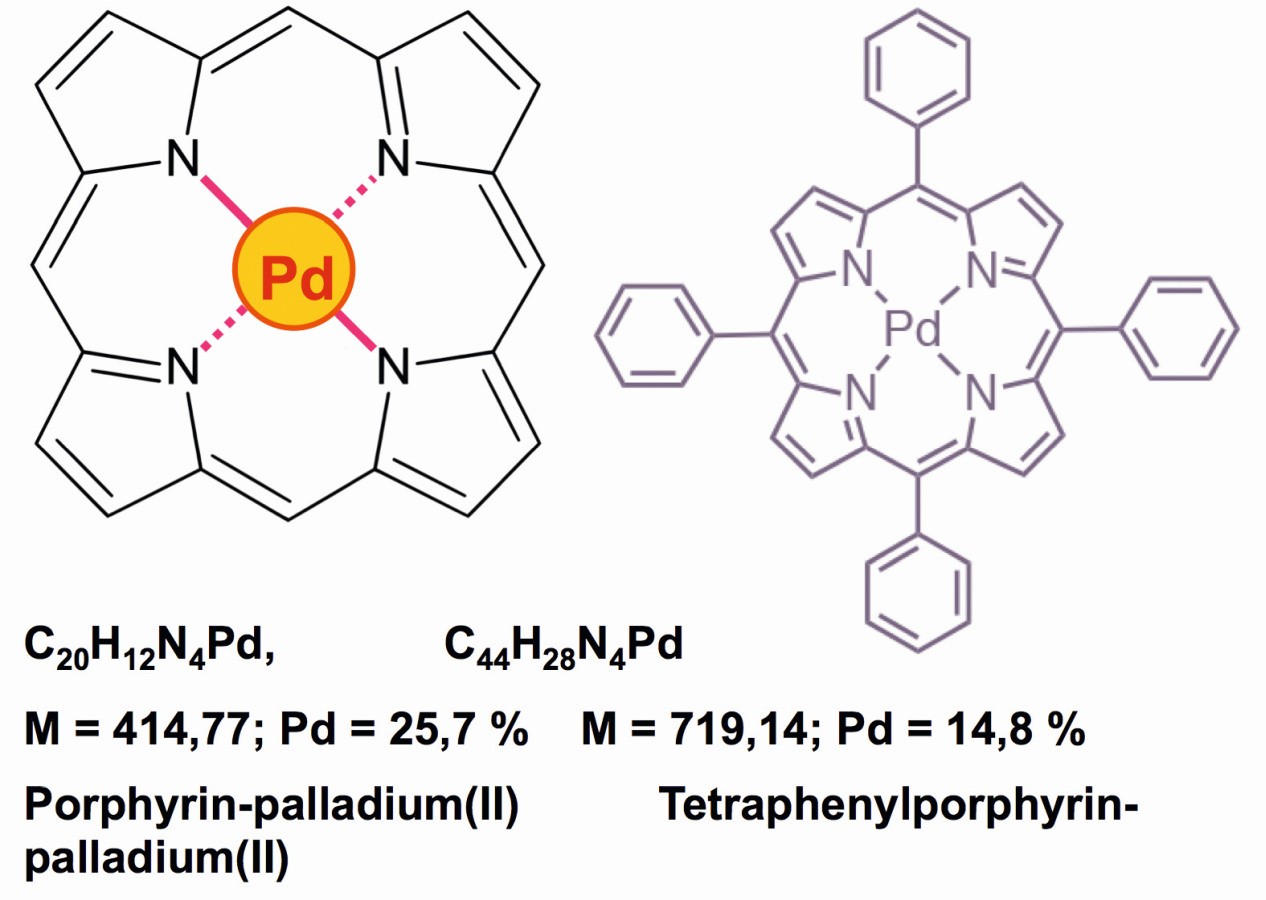 Fig. 22: Red porphyrin compounds of palladium
Fig. 22: Red porphyrin compounds of palladium
7 Palladium recycling
Prerequisites for extensive recycling are
- Recycling-friendly designs
- Information on the resource conservation of palladium-containing scrap and dross
- Optimized collection logistics
- Good infrastructure of reprocessing plants
- Process optimization according to the best available technology
- Resource conservation as a priority
With all rare element resources, the economic component is unfortunately the top priority. This leads to considerable losses: due to convenience, ignorance, technical and logistical inadequacies and accidents.
Figure 23 shows a balance of the material flows for the reprocessing of palladium residues from production and consumers for 2015. The largest proportion of palladium returns is accounted for by far by catalytic converters, electronic components and end-of-life vehicles [51].
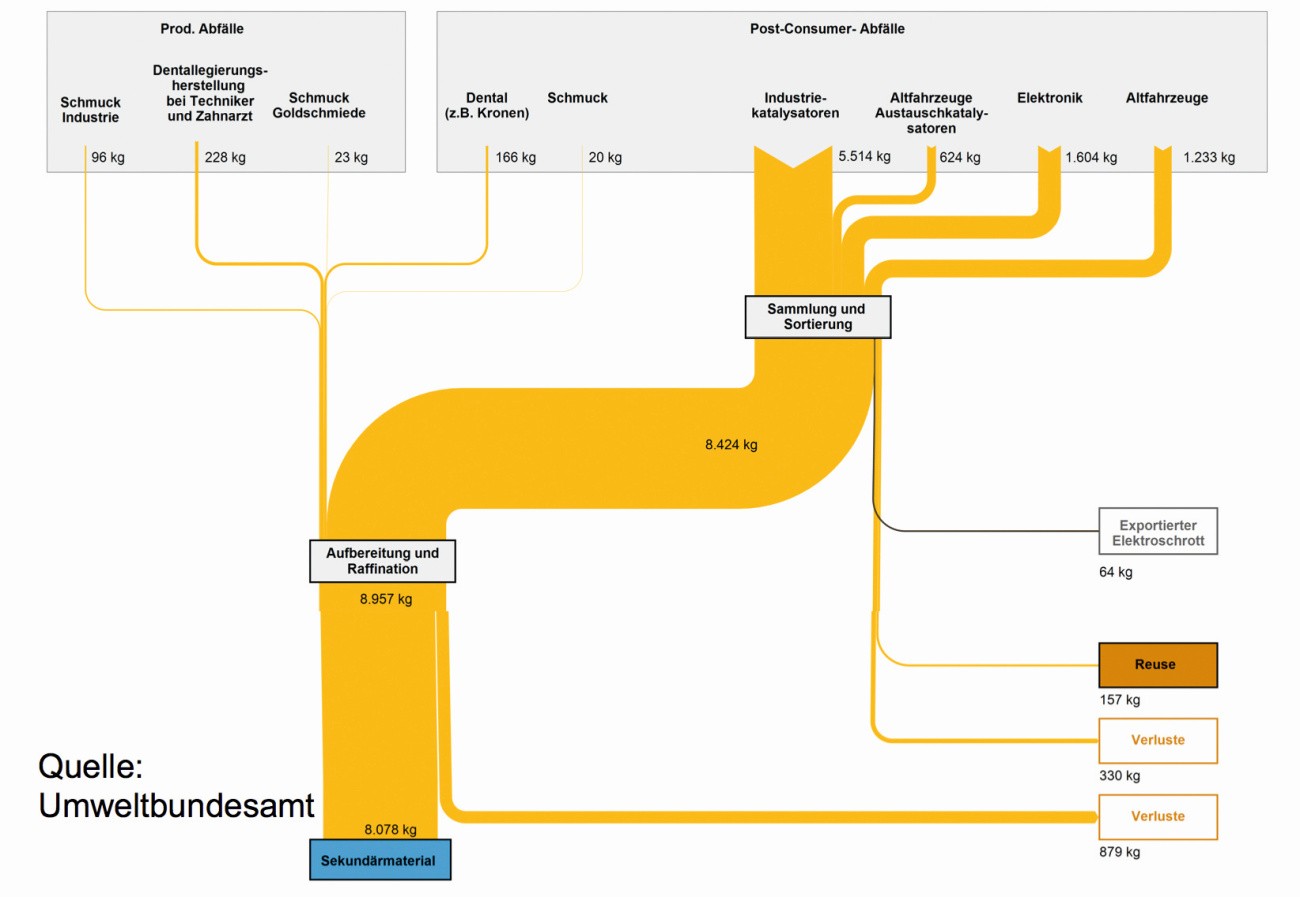 Fig. 23: Material flows of palladium recycling in Germany in 2015
Fig. 23: Material flows of palladium recycling in Germany in 2015
On the long routes of palladium use alone, 10 to 30 % of the metal disappears: some of the chemical catalysts on the various carriers, such as activated carbon, calcium carbonate, etc., are lost in the product, while many of the electronic components end up as residual waste or in landfills, where the palladium is lost forever. In the case of car exhaust catalytic converters, the extent to which the "washcoat" containing the precious metals is swept out of the exhaust depends on the driving style of the vehicle owner. In the case of lowered cars with a few bumps of the underbody on kerbs and road unevenness as well as speeds above 200 km/h, there is certainly not much catalytic converter ceramic left in the exhaust system.
In 2016, the Federal Ministry for the Environment, BMU, commissioned research into the resources for 2020. Based on the reference and average values, the products analyzed resulted in a total expected palladium quantity of 14,201 kg in 2020. The range of the determined quantity extends from 13,737 to 14,664 kg. The largest contributions come from used industrial catalytic converters at 10,307 kg, followed by automotive catalytic converters at 3,578 kg. Data centers contribute only slightly to palladium recycling with around 316 kg [52].
Precious metal separators have separation processes for processing palladium residues that are more or less adapted to the input material. In some cases, high-temperature chlorination of alloys at 800 °C is sufficient to remove the base metal traces. Hydrazine precipitation of the precious metals or ion exchange processes, for example, are used for precious metals that are strongly accompanied by base metals.
If other precious metals are present in addition to palladium, specific or general separation processes are used [53-57].
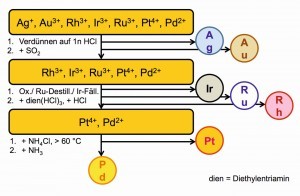
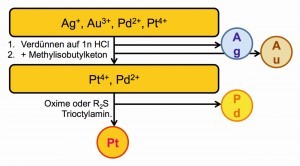
This can be a precious metal separation process with predominantly selective precipitation (Fig. 24) or separations via liquid-liquid extractions between the media water and gasoline or other organic solvents that are not miscible with water (Fig. 25).
Palladium is usually separated using the sparingly soluble ammonium hexachlorido-palladate(IV), (NH4)2[PdCl6]/Cl2, by oxidation with chlorine gas. Strangely enough, these precipitations always proceeded without incident.
At the research institute in Freiberg, analogous experiments showed that nitrogen trichloride, NCl3, an extremely explosive compound, is formed as a side reaction of this precipitation [58]. The nitrogen trichloride, which is oily at room temperature, collects at the bottom of the reactor with a density of 1.64, but with a boiling point of 71 °C it is almost as volatile as alcohol.
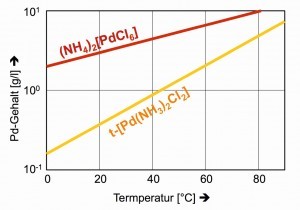
The poor solubilities of the classic precipitates in the separation path, the dark red (NH4)2[PdCl6] and the yellow trans-[Pd(NH3)2Cl2], are only satisfactory at lower temperatures (Fig. 26).
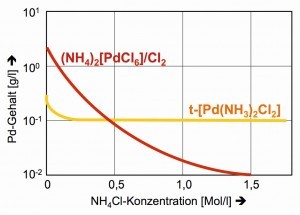
The solubility of hexachloropalladate(IV) can be further reduced by ammonium chloride, a common method of salting out that naturally fails with the neutral complex [Pd(NH3)2Cl2] (Fig. 27).
The thermal decomposition of diammine dichloride palladium(II) in the temperature range of 600 to 800 °C guarantees a reliable method for obtaining coarsely crystalline palladium, also known as "palladium sponge" (Fig. 28).
Particularly in research institutes and smaller companies, technicians are tempted to carry out precipitations with formic acid, formate, hydrazine or boranate without safety precautions when smaller quantities of palladium salt are used. On the one hand, the fine palladium metal powders are pyrophoric due to the absorption of hydrogen, and on the other hand, the reducing agents themselves release hydrogen, such as formic acid, whose hazardous substance properties are a challenge in themselves [59]:
H2[Pd2+Cl4] + H-C2+O-OH→ Pd0 + C4+O2 + 4 HCl
H-CO-OH →CO2 + H2 (palladium-catalyzed reaction!).
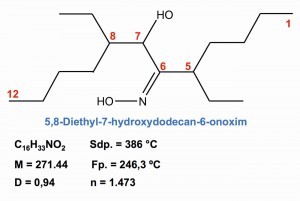 Fig. 29: Extraction of palladium with oximes In the selective separation of palladium from other metals, extractions with oximes and dialkyl thioethers have become increasingly important. One extraction chemical specially developed for palladium is 5,8-diethyl-7-hydroxydodecane-6-onoxime, whose formal molecular structure and properties are shown in Figure 29 .
Fig. 29: Extraction of palladium with oximes In the selective separation of palladium from other metals, extractions with oximes and dialkyl thioethers have become increasingly important. One extraction chemical specially developed for palladium is 5,8-diethyl-7-hydroxydodecane-6-onoxime, whose formal molecular structure and properties are shown in Figure 29 .
Among the di-n-alkyl thioethers, the di-n-hexyl and di-n-octyl thioethers are mainly used in industry. As can be seen in Table 1, the solubility in the series of thioethers decreases with increasing molecular weight; on the other hand, the flash points are in the safe range at high molecular weights.
After re-extraction with concentrated hydrochloric acid, the tetrachloridopalladate(II) is again present, which is precipitated with ammonia as [Pd(NH3)2Cl2] and calcined.
8 The future of palladium
Palladium has emerged as the "shining star" among metals for investors, who have enjoyed double-digit growth rates in recent years. Rising demand, stricter environmental regulations for vehicles in China and the switch from diesel to petrol cars in Europe point to a rosy future for palladium. The limited palladium production, together with the undersupply in recent years, may also drive prices up further.
The positive forecasts extend to 2024, when the palladium price is expected to reach USD 3400/troz (31.1g) [60].
Other stock market analysts see the slump in the global automotive industry as the end of the palladium boom. As almost 85% of globally mined palladium ends up in the catalytic converters of petrol vehicles, demand for this precious metal has fallen drastically due to the virtual standstill of the car market during the coronavirus lockdown. The palladium price has so far recovered only slightly from the sharp drop in price from USD 2,800 to 1,600 per troy ounce.
Fears that less palladium will be needed due to the increase in electric cars are likely to be unfounded for the time being. Despite subsidies, the market for battery-powered vehicles is only taking off very slowly. The future of hybrid vehicles looks brighter. And they even require 10 to 15 % more palladium than conventional vehicles [61].
Literature
[1] Weeks, M.E.: Discovery of the Elements, Journal of Chemical Education, 6th ed. (1956) 409
[2] https://de.wikipedia.org/wiki/(2)_Pallas
[3] Wollaston, W.H.: On a New Metal, Found in Crude Platina, Phil. Trans. R. Soc. 94 (1804) 419-430
[4] Graham, Th.: On the relation of hydrogen to palladium, Proceedings of the Royal Society of London. 17 (1869) 212-220
[5] Phillips, F.C.: Am. Chem. J., 16 (1894) 255-277
[6] Precious Metals Pocketbook, Degussa (1967) 72
[7] http://forkat.anorg.chemie.tu-muenchen.de/biblio/texte/pdf
[8] Precious Metals Pocketbook, Degussa, Hüthig (1995)
[9] https://www.wotech-technical-media.de/womag/ausgabe/2013/10/21_w_edelmetalle_schade_10j2013/21.php
[10] Precious Metals Pocketbook, Degussa (1967) 70
[11] https://de.wikipedia.org/wiki/Platinmetalle/Tabellen_und_Grafiken
[12] https://de.wikipedia.org/wiki/Palladium
[13] Holleman; Wiberg: Inorganic Chemistry, vol. 2, 103rd ed. (2017) 2039
[14] https://de.wikipedia.org/wiki/Thioether
[15] https://www.kettner-edelmetalle.de/Palladium
[16] https://www.scheideanstalt.de/aktuelle-ankaufskurse/palladium/
[17] https://www.handelsblatt.com/finanzen/maerkte/devisen-rohstoffe/rohstoffe-warum-der-palladium-preis-seit-jahresbeginn-explodiert/
[18] https://feingoldhandel.de/palladium-das-silberweisse-edelmetall
[19] Aston, J.G.; Mitacek, P., Jr: Structure of hydrides of palladium, Nature, London, UK, 195 (1962) 70-71
[20] https://www.pv-magazine.de/2017/09/01/solarstrom-wirtschaftlich-in-wasserstoff-umwandeln/
[21] https://www.seilnacht.com/Lexikon/swasser.html
[22] https://de.wikipedia.org/wiki/Anthrachinon-Verfahren
[23] Kickelbick, G.: Chemistry for Engineers, Pearson Germany, (2008) 155
[24] https://de.wikipedia.org/wiki/Herbert_Lindlar
[25] Rosenmund, K.W.: Über eine neue Methode zur Darstellung von Aldehyden, 1. Mitteilung, Berichte der deutschen chemischen Gesellschaft. 51 (1918) 585-593
[26] Steinborn, D.: Fundamentals of organometallic complex catalysis, Teubner, Wiesbaden (2007) 283-292
[27] https://www.chemhui.com/pro/37668
[28] Yang, J.; Liu, J.; Neumann, H.; Franke, R.; Jackstell, R.; Beller, M.: Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes, Science, 366 (2019) 1514
[29] https://goetze-gold.de/halbzeuge-zuschnitt/blech/platin-und-palladium/
[30] https://www.wegold.de/produkte/legierungen/palladium-basis-legierungen/676-wegold-dg-dentallegierung
[31] https://de.wikipedia.org/wiki/Dentallegierungen
[32] https://www.edelmetalle.org/medizintechnik/
[33] https://www.aerogen.com/de/technologie/
[34] https://patents.google.com/patent/DE60003490T2/de
[35] https://www.edelmetalle.org/elektronik/
[36] Buth, M.A.: Palladium from PC and electronics, special volume, adrenalinemedia (2012)
[37] RÖMPP, Lexikon Chemie, 8th edition, vol. 4 (1985) 2973
[38] https://de.wikipedia.org/wiki/Ilja_Iljitsch_Tschernjajew
[39] https://de.wikipedia.org/wiki/Trans-Effekt
[40] Tanaka, M.: Structure of Bis(tri-tert-butylphosphine)palladium(0), Acta Cryst., C48 (1992) 739-740
[41] He, L.-Y.: Bis(tri-tert-butylphosphine)palladium(0) [Pd(t-Bu3P)2], Synlett, 26/6 (2015) 551-852
[42] https://shop.jentner.de/galvanische-elektrolyte/edelmetall-elektrolyte/Palladiumbad/
[43] Talgner, F., Myers, M.; Schmidt, H.: Galvanic silver-palladium as a contact surface, Galvanotechnik, Leuze-Verl., 05 (2017) 910
[44] Hasenpusch, W.: Explosivity of tetramine metal nitrates, J. prakt. Chem, Chemiker-Zeitung 335 (1993) 193-196
[45] Chemiker-Fachausschuss d. Gesellschaft Metall und Erz: Analyse der Metalle I: Schiedsverfahren, Springer-Verl., Berlin (1942) 184
[46] Burger, K., Dyrssen, D.; Johansson, L.; Norén, B.; Munch-Petersen, J.: On the Complex Formation of Palladium with Dimethylglyoxime, Acta Chemica Scandinavica, 17 (1963) 1489
[47] Fries, J.; Getrost,H.: Merck, E., Darmstadt: Organische Reagenzien für die Spurenanalyse" (1975) 285
[48] https://en.wikipedia.org/wiki/Palladium(II)_bis(acetylacetonate)
[49] Setsune, J.: Palladium chemistry in recent porphyrin research Journal of Porphyrins and Phthalocyanines, 08/01(2004) 93-102
[50] To, W.; Liu, Y.; Lau, T.; Che, C.: A Robust Palladium(II)-Porphyrin Complex as Catalyst for Visible Light Induced Oxidative C-H Functionalization, Chemistry - A European Journal, 19/18 (2013)
[51] https://www.umweltbundesamt.de/platin-palladium#hinweise-zum-Recycling
[52] https://www.bmu.de/fileadmin/Daten_BMU/Pools/Forschungsdatenbank/fkz_3711_93_339_substitutionspotenziale_Metalle_bf.pdf
[53] Precious Metals Pocketbook, Degussa AG, Frankfurt, Hüthig- Verl. Heidelberg (1995) 48
[54] Doduco data book, Pforzheim (1974) 351
[55] Bertau, M.; Müller, A.; Fröhlich, P.; Katzberg, M.: Industrielle Anorganische Chemie, 4th edition, Wiley-Verl., Weinheim (2013) 363-371
[56] Metal Research and Development, Degussa AG (1991) 33-44
[57] Annual Seminar Proceedings of the International Precious Metals Institute, IPMI, USA
[58] Knothe, M.; Hasenpusch, W.: The formation of explosive chlorine-nitrogen compounds during the processes of precious metals separation, Journal of Hazardous Materials, 56 (1997) 137-148
[59] https://de.wikipedia.org/wiki/Ameisensaeure
[60] https://capital.com/de/palladium-preisentwicklung
[61] https://www.boerseonline.de/nachrichten/rohstoffe/voruebergehende-flaute-warum-der-palladium-preis-bald-wieder-steigen-duerfte-102934254