After platinum, palladium is also one of the most sought-after, versatile metals of the six platinum group elements, which also include rhodium, iridium, ruthenium and osmium. They are all successfully used as catalysts, as metals or in the form of their chemical compounds. White gold would be inconceivable in the jewelry sector without palladium, and the permeability of hot palladium walls to hydrogen opens up a range of technical applications. Due to the high physical incorporation of hydrogen into the metal lattice of palladium powders, it is favored as a storage medium, but also turns into a pyrophoric bomb when exposed to air and chlorine. Above all, the use of palladium in three-way catalytic converters for motor vehicles increased demand, mine production and the price.
1 The history of a precious metal
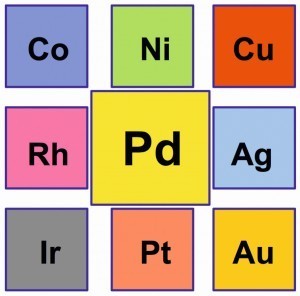 Fig. 1: Palladium and its neighbors in PSEIn1802,theEnglish physician and chemist William Hyde Wollaston (1766-1828) discovered the new element in a South American platinum ore after he dissolved platinum, which the Spanish scholar and admiral Antonio de Ulloa had already described in 1748 [1], in aqua regia and precipitated it as ammonium hexachloridoplatinate(IV), in the remaining mother liquor as a pure metal. This was done in an alkaline solution with mercuric cyanide, Hg(CN)2, and subsequent calcination.
Fig. 1: Palladium and its neighbors in PSEIn1802,theEnglish physician and chemist William Hyde Wollaston (1766-1828) discovered the new element in a South American platinum ore after he dissolved platinum, which the Spanish scholar and admiral Antonio de Ulloa had already described in 1748 [1], in aqua regia and precipitated it as ammonium hexachloridoplatinate(IV), in the remaining mother liquor as a pure metal. This was done in an alkaline solution with mercuric cyanide, Hg(CN)2, and subsequent calcination.
As the Bremen astronomer and physician Heinrich Wilhelm Olbers had discovered a new planetoid in the asteroid belt shortly beforehand in 1802 [2] and named it "Pallas" after the Greek goddess "Pallas Athena", Wollaston suggested "palladium" for his new element [3].
As early as 1866, Thomas Graham (1805-1869) noted the astonishing storage capacity of finely dispersed palladium for hydrogen, which can hold around 900 times its own volume of hydrogen gas at room temperature and atmospheric pressure [4].
In 1894, the American chemist Francis Clifford Phillips (1850-1920) discovered the stoichiometric oxidation of ethene, H2C=CH2, to acetaldehyde, H3C-CH=O, in the presence of palladium(II) chloride, PdCl2, when he was investigating the oxidation of naturally occurring hydrocarbons [5]. A process that Wacker-Chemie later used to produce one million tons of acetaldehyde per year with the subsequent product acetic acid, CH3COOH.
2 Chemical and physical properties
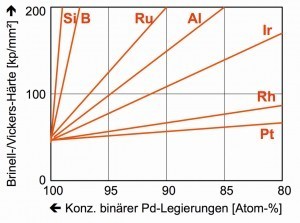 Fig. 2: Hardness curve of alloyed palladium alloysPalladiumwith atomic number 46 consists of six stable isotopes, which together have an atomic weight of 106.42. In the periodic table of elements, it ranks above the "heavy platinum group metals" iridium and platinum with densities of 22.56 and 21.45 g/cm3 (20 °C) and gold with a density of 19.32 g/cm3. The elements rhodium and silver flank palladium. Together with rhodium, palladium is one of the "light platinum group metals" with densities of 12.38 and 11.99 g/cm3. The density of silver is approximately the same at 10.49 g/cm3. Above palladium are the base transition metals cobalt, nickel and copper (Fig. 1).
Fig. 2: Hardness curve of alloyed palladium alloysPalladiumwith atomic number 46 consists of six stable isotopes, which together have an atomic weight of 106.42. In the periodic table of elements, it ranks above the "heavy platinum group metals" iridium and platinum with densities of 22.56 and 21.45 g/cm3 (20 °C) and gold with a density of 19.32 g/cm3. The elements rhodium and silver flank palladium. Together with rhodium, palladium is one of the "light platinum group metals" with densities of 12.38 and 11.99 g/cm3. The density of silver is approximately the same at 10.49 g/cm3. Above palladium are the base transition metals cobalt, nickel and copper (Fig. 1).
The proximity to platinum results in the same usual oxidation states of +2 and +4. With a Mohs hardness of only 4.75, i.e. comparable to a mussel shell, pure palladium appears relatively soft. The hardness of palladium increases rapidly when even small amounts of foreign metal are added (Fig. 2). This effect is very important for technical applications [6].
Particular attention should be drawn to the high hydrogen uptake of nanoscale palladium powder: H2 can be stored in the metal lattice up to 3,000 times its volume (Fig. 3).
Heated tubes made of palladium are comparable to a sieve through which hydrogen can be easily separated from other gases, and the activation of hydrogen, which is of great importance for the hydrogenation of organic double and triple bonds, can also be understood in this way.
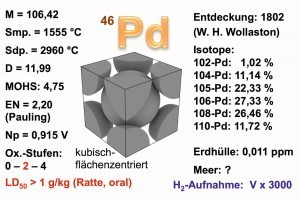 Fig. 3: Properties of the palladium metal The main oxidation state of palladium is +2, as for example in diamminedichlorido-palladium(II), [Pd(NH3)2Cl2]. Strong oxidizing agents, such as chlorine, are required for the +4 oxidation state. Nevertheless, hexachloropalladate(IV) decomposes back into divalent palladium at temperatures above 60 °C, an effect that is also used in the separation process of platinum group metals, PGM:
Fig. 3: Properties of the palladium metal The main oxidation state of palladium is +2, as for example in diamminedichlorido-palladium(II), [Pd(NH3)2Cl2]. Strong oxidizing agents, such as chlorine, are required for the +4 oxidation state. Nevertheless, hexachloropalladate(IV) decomposes back into divalent palladium at temperatures above 60 °C, an effect that is also used in the separation process of platinum group metals, PGM:
H2[PdCl6] - 60 °C → H2[PdCl4] + Cl2.
The surface of palladium [7] shows a certain carotte-shaped surface roughness even under the electron microscope (Fig. 4 A).
Palladium powders are available in a wide range of specifications, habitus and grain size distributions, depending on the application [8]. Different reducing agents, parameters and reaction conditions result in different powder characteristics: crystalline, platy, spherical (Fig. 4 B).
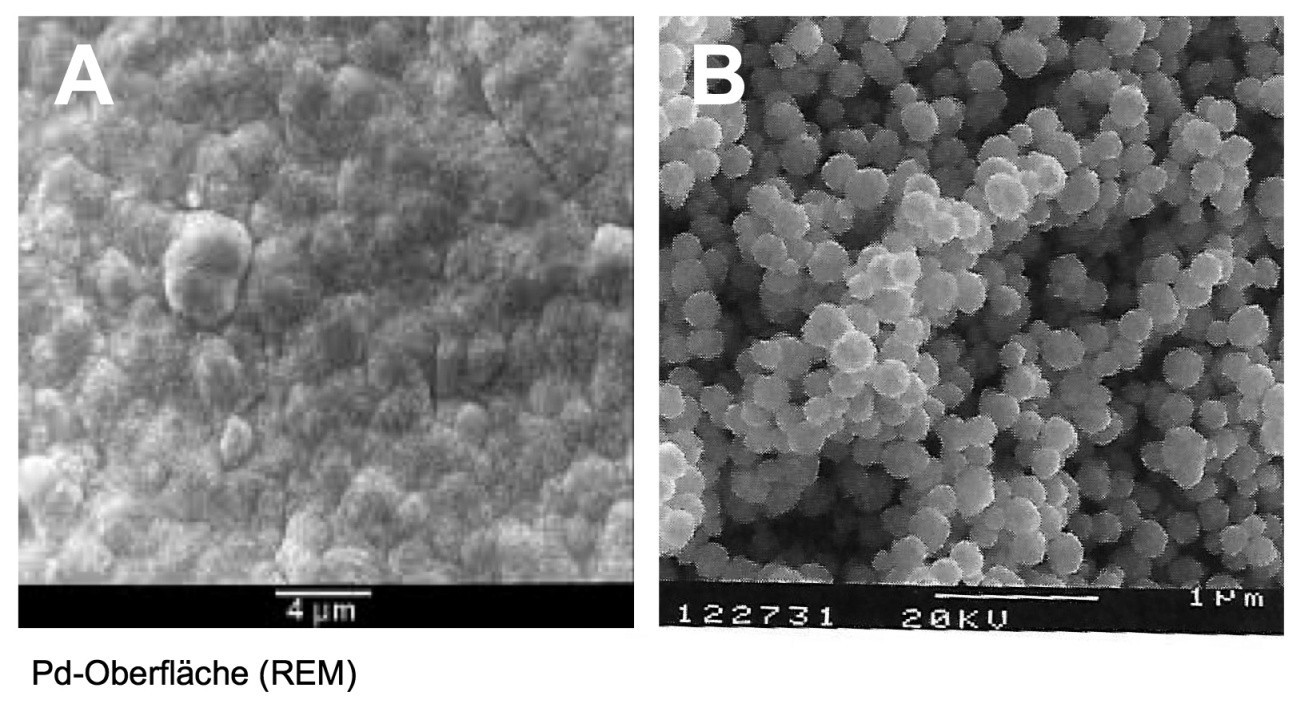 Fig. 4: SEM images of a palladium surface (A) and palladium powder for multilayer capacitors (B)
Fig. 4: SEM images of a palladium surface (A) and palladium powder for multilayer capacitors (B)
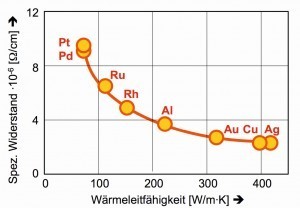
In terms of thermal conductivity and conductivity or resistance to electric current [9], palladium, together with its "big brother" platinum, is clearly inferior to the classic conductive metals silver, copper and aluminum (Fig. 5).
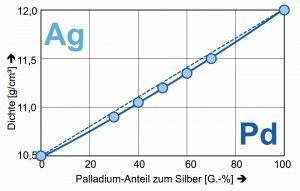
What is interesting about the binary alloys of palladium with silver or gold is the almost straightforward relationship between the weight proportions and the density (Fig. 6). This makes it easy to determine the composition of the binary alloys [10].
3 Occurrence and extraction of palladium
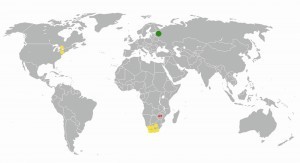 Fig. 7: Global palladium extraction in 2005 (Russia: 143 t; green dot); yellow dot: approx. 15 t, red dot: approx. 1.5 tAlthoughthe palladium deposits in the earth's mantle appear to be at a very low concentration of 0.011 ppm, not to mention the barely determinable concentrations in seawater, it is found associated with other PGMs, solid alloyed, as an oxide or sulphide, in some countries. The world map with the noteworthy locations reveals the rarity of this element, which apparently cannot be found in the entire Asian region (Fig. 7).
Fig. 7: Global palladium extraction in 2005 (Russia: 143 t; green dot); yellow dot: approx. 15 t, red dot: approx. 1.5 tAlthoughthe palladium deposits in the earth's mantle appear to be at a very low concentration of 0.011 ppm, not to mention the barely determinable concentrations in seawater, it is found associated with other PGMs, solid alloyed, as an oxide or sulphide, in some countries. The world map with the noteworthy locations reveals the rarity of this element, which apparently cannot be found in the entire Asian region (Fig. 7).
Russia, South Africa and Canada can be described as the frontrunners (Fig. 8), followed by the USA and Zimbabwe, which brought 14 and 12 tons of palladium onto the global market in 2017. According to estimates from 2011, South Africa has the largest reserves in the entire PGM sector at 63,000 tons [11, 12].
In Russia and South Africa, these are the copper-nickel-chromium gravels on both sides of the Ural Mountains and the palladium deposits in the Bushfield area. In Canada, the copper-nickel-iron gravels in the Sudbury Basin of Ontario contain some palladium [13].
Some intermetallic phases also occur as palladium minerals, as cited in the official "List of Minerals":
- Atokite: Pd3Sn; D = 14.19; Mohs hardness = 4.5
- Cabriite: Pd2SnCu; D = 11.1; Mohs hardness = 4.0 - 4.5
- Paolovite: PdSn2; D = 11.08; Mohs hardness = 4.5
- Potarite: PdHg; D = 14.88; Mohs hardness = 3.5
- Stannopalladinite: (Pd/Cu3) Sn2; Mohs hardness = 4.5 - 5.0
- Zvyagintsevite: Pd3Pb; D = 13.32; Mohs hardness = 4.5.
The PGMs are separated by washing, leaching and electrolysis processes of the copper, nickel and chromium ores, whereby the precious metals are concentrated as anode slimes. Ores with a high silver content can be enriched in lead or other collector metals in shaft or rotary kilns. The oxidized "smooths" (oxides) can be easily separated from the smelting surface and the PGMs can be recovered from the anode slimes of the silver electrolysis.
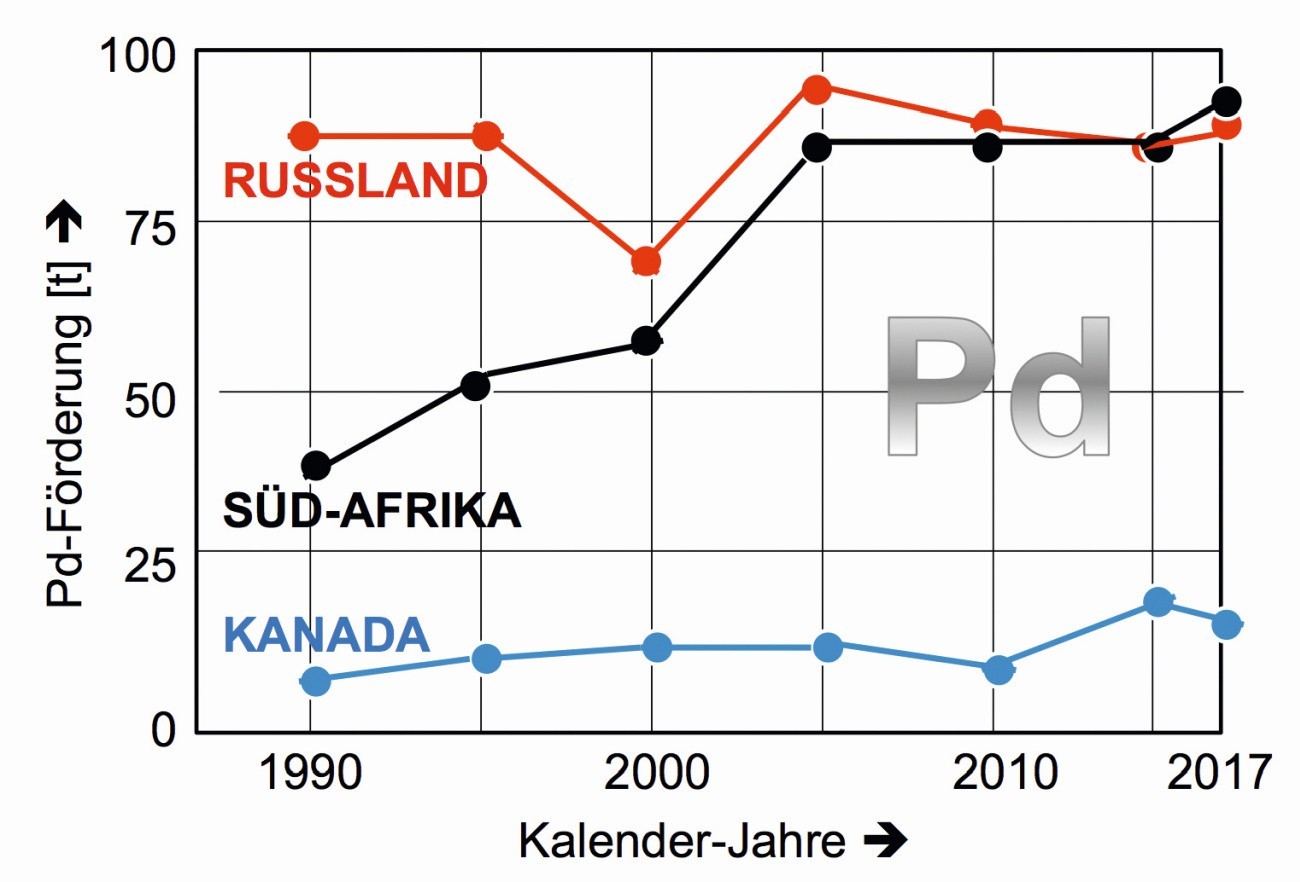 Fig. 8: Regional palladium production 1990 to 2017 in the three largest producing countries
Fig. 8: Regional palladium production 1990 to 2017 in the three largest producing countries
Extractions with the thioethers dihexyl and dioctyl sulphide have become established for separating the palladium from the other PGMs, partly due to the risks associated with ammonia precipitation [14]. The properties within the thioether series from di-n-methyl to di-n-octyl ether are shown in Table 1.
|
Name |
Formula |
M |
Smp. [°C] |
Sdp. [°C] |
Fp [°C] |
D [g/cm3] (20 °C) |
n (20 °C) |
LH2O [g/l] (20 °C) |
H-phrases |
|
Di-n-methyl-S. |
C2H6S |
62,14 |
-98 |
37 |
-45 |
0,85 |
1,4355 |
2,0 |
225-319 |
|
Di-n-ethyl-S. |
C4H10S |
90,19 |
-104 |
92 |
-10 |
0,84 |
1,442 |
3,2 |
225-315-319 |
|
Di-n-propyl-S. |
C6H14S |
118,2 |
-103 |
143 |
28 |
0,838 |
1,4487 |
0,35 |
225-315-319-412 |
|
Di-n-butyl-S. |
C8H18S |
146,3 |
-80 |
189 |
62 |
0,84 |
1,452 |
315-319-335 |
|
|
Di-n-hexyl-S. |
C12H26S |
202,4 |
260 |
116 |
0,85 |
1,4587 |
315-318-335 |
||
|
Di-n-octyl-S. |
C16H34S |
258,5 |
-1 |
160 |
0,844 |
0,000.005 |
315-319-335 |
The advantages of di-n-hexyl and di-n-octyl thioethers in terms of low solubility in water and high flash point as favorable parameters for extraction with hydrophobic solvents, such as gasoline, are particularly striking.
4 The palladium market
 Fig. 9: Soviet palladium coin Palladium is available as bars or coins (Fig. 9) on the investment market, where it can be purchased from banks. With a purity of 999.9 parts per thousand, it is often used for jewelry processing in alloys of 950 parts per thousand palladium or even lower. Demand for the metal has risen steadily in recent years. As a result, the importance of this precious metal in the diversification of the investment spectrum is also growing. As pure palladium is too soft for many pieces of jewelry, they contain a lower proportion of it. Bars and coins are therefore recommended as an investment.
Fig. 9: Soviet palladium coin Palladium is available as bars or coins (Fig. 9) on the investment market, where it can be purchased from banks. With a purity of 999.9 parts per thousand, it is often used for jewelry processing in alloys of 950 parts per thousand palladium or even lower. Demand for the metal has risen steadily in recent years. As a result, the importance of this precious metal in the diversification of the investment spectrum is also growing. As pure palladium is too soft for many pieces of jewelry, they contain a lower proportion of it. Bars and coins are therefore recommended as an investment.
The Canadian "Maple Leaf" weighing one ounce cost 2326.59 euros on 3.8.2020 including VAT, excluding shipping. The 1 ounce bar is offered by precious metal refineries Umicore, Heraeus and Degussa for €2407.36, the 500g bar for €36,416.27 and the 1 kg palladium bar for €71,012.57 [15].
While palladium is relatively cheap to buy as a coin, but only in a few sizes, the bars are available in 10 g, 20 g, 1 ounce (31.1 g), 50 g, 100 g, 500 g and 1000 g. The difference between the selling and buying price of precious metals often leads to confusion: the buying price for bankable 999.5 palladium in the form of bars, slabs or coins is currently 55.44 euros/gram (all prices refer to mid-2020).
For recycled or fused palladium from jewelry, dental gold or "scrap" (Fig. 10), the purchase prices are staggered at
- 41.15 euros up to 10 g Pd content
- 41.72 euros up to 500 g Pd content and
- 44.01 euros over 500 g Pd content.
This means, however, that the 1 kg Pd bar just purchased for 71,012.57 euros can currently be returned for 55,440 euros. The investor must therefore hope for a considerable increase in value [16].
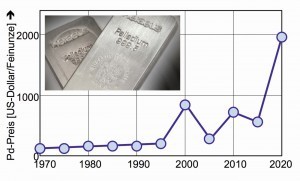
In any case, palladium is currently experiencing a rapid price increase (Fig. 11) - even precious metal traders are amazed. A price explosion that gold has never experienced in this way. At the beginning of 2020, the troy ounce was still at 1,300 dollars, while the price of palladium shot through the 2,000-dollar ceiling in the middle of the year.
According to experts, this was due to relatively restrained palladium production, while demand from the automotive industry grew steadily. Today, demand in the automotive industry is at 80% after manufacturers replaced a large proportion of the platinum in car exhaust catalytic converters with palladium, which was relatively inexpensive in 2009. There is currently a very high supply deficit [17].
5 Use of palladium
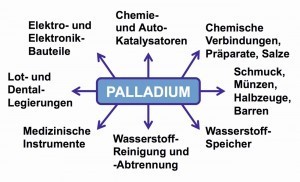 Fig.12: Main areas of application for palladium The use of palladium is similar to that of platinum in many areas due to its comparable properties. In industry, it is used to temper metals, in hydrogen technology as a storage medium or as a catalyst. Jewelry made of palladium alloyed with gold, which makes the gold color disappear, is popular. These white gold alloys are also used in medical and dental technology.
Fig.12: Main areas of application for palladium The use of palladium is similar to that of platinum in many areas due to its comparable properties. In industry, it is used to temper metals, in hydrogen technology as a storage medium or as a catalyst. Jewelry made of palladium alloyed with gold, which makes the gold color disappear, is popular. These white gold alloys are also used in medical and dental technology.
As an economic raw material, the price of palladium is more closely linked to the current economic situation than gold, the price of which is still more closely linked to investor demand.
The gray precious metal has proven to be very attractive as an investment in the form of bars or coins, especially in recent years [18].
Figure 12 provides an overview of the main areas of application for palladium.
5.1 Hydrogen technology
Palladium has the highest absorption capacity of all elements for hydrogen. This fundamental discovery goes back to Thomas Graham in 1869. At room temperature, it can bind 900 times its own volume, finely dispersed black palladium powder 1200 times its own volume and colloidal palladium solutions 3000 times their own volume. The "compound" formed after hydrogen saturation in the metal lattice can be approximated as a palladium hydride with the approximate composition Pd2H[19].
Since palladium has the property of storing hydrogen up to several thousand times in its metal lattice, it became attractive for the storage and transportation of this gas. Solar power plants in the equatorial regions can collect the sustainably produced energy carrier hydrogen and make it available across the entire globe [20].
Instead of using fuels derived from fossil fuels such as petrol, diesel or natural gas, a car can also run on hydrogen.
Compared to the other vehicle drives mentioned, the hydrogen car is significantly more environmentally friendly. A hydrogen vehicle that is equipped with an electric motor and obtains its energy from the conversion of chemical energy in the fuel cells is even more sustainable. However, experts consider electric cars to be the most favorable drive variant if they are powered by electricity from wind power plants [21].
5.2 Use as a catalyst
Palladium serves as a catalyst for many technical processes. The majority of these are partial or complete hydrogenations. For example, the production of hydrogen peroxide using the "anthraquinone process" also takes place in the hydrogenation step with chemically precipitated palladium powder as a catalyst [22]:
Anthraquinone + H2/Pd → Anthrahydroquinone ... + O2 →
anthraquinone + H2O2.
In the "three-way catalytic converter" in vehicle combustion engines, palladium, in addition to platinum in some cases, is responsible for the two paths:
- Oxidation of carbon monoxide to carbon dioxide and
- oxidation of hydrocarbons to carbon dioxide and water.
Rhodium is mainly responsible as a catalyst for the reduction of nitrogen oxides to nitrogen [23].
In order not to fully hydrogenate alkynes (A), but only up to the alkene (B), "Lindlar catalysts" are used, a process developed by the British-Swiss chemist Herbert Lindlar (1909-2009) at the pharmaceutical company Hoffmann-La Roche. These are calcium carbonate carriers with 5 % reduced palladium metal, phlegmatized ("poisoned") by sulphur or similar [24]:
A. R-C = C-H + 2 H2 → R-CH2-CH3
(without Lindlar catalyst
B. R-C = C-H + H2 → R-CH = CH2
(with Lindlar catalyst).
A phlegmatized hydrogenation also takes place using the "Rosenmund catalyst", in which palladium was deposited on barium sulfate. In this way, hydrogenation processes can be carried out in which the hydrogen reduces an acyl chloride to an aldehyde. This reaction was first described by Karl Wilhelm Rosenmund (1884-1965) in 1918 [25]. Barium sulphate serves as a metal carrier, limits the activity of the palladium and thus prevents a reduction beyond the aldehyde stage.
Palladium is used as a chloride in the "Wacker-Hoechst process", in which ethylene (ethene) is selectively oxidized to acetaldehyde. The following compounds, among others, occur as intermediates [26]:
- Trichloridoethylene palladinate(II) anion:
[Cl3Pd---C2H4]- ,
- trans-dichlorido-hydroxido-ethylene palladate(II):
[Cl2(OH)Pd---C2H4]-,
- trichlorido-2-hydroxyethyl palladium(II):
[Cl3Pd-CH2-CH2-OH]2-.
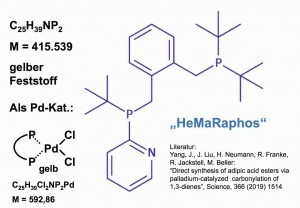 Fig. 13: Molecular structure of "HeMaRaphos", a phosphine ligand for a palladium catalyst for the production of adipic acid derivativesOnthe way to a new process for the production of adipic acid, researchers from academia and industry achieved product yields of 95 % by catalytic carbonylation of butadiene with the aid of a special palladium-phosphine catalyst:
Fig. 13: Molecular structure of "HeMaRaphos", a phosphine ligand for a palladium catalyst for the production of adipic acid derivativesOnthe way to a new process for the production of adipic acid, researchers from academia and industry achieved product yields of 95 % by catalytic carbonylation of butadiene with the aid of a special palladium-phosphine catalyst:
H2C=CH-CH=CH2 + 2 CO + 2H2O→ HOOC-C4H8-COOH.
The palladium catalyst is a new compound with a special phosphine ligand called "HeMaRaPhos" [27] (Fig. 13).
The reaction takes place at 120 °C and a pressure of 40 bar within 24 hours in butanol [28].
Experts believe that the new process for the production of adipic acid and its derivatives has a successful future, as manufacturers of adipic acid derivatives produce several million tons annually, which are used for further processing into plasticizers, perfumes, lubricants, solvents, active pharmaceutical ingredients and, above all, nylon.
5.3 Semi-finished products for the jewelry industry
As with the other common precious metals, refineries and precious metal traders also sell semi-finished palladium products, e.g. for jewelry manufacturers. As with other precious metals, palladium is available in the form of wires, sheets, strips, rods, tubes in various diameters and profiles, solder and cast alloys. For the most part, however, these semi-finished products are not available from stock, but have to be produced promptly according to customer requirements. Consequently, the specified fabrications and blanks are excluded from direct exchange.
Jewelry manufacturers particularly value palladium and white gold for rings, earrings and necklaces set with diamonds. Like platinum, palladium is also highly resistant to corrosion and its hardness is significantly higher than that of gold. Palladium retains its shine and dark grey color permanently and does not tarnish.
Palladium is more resistant to abrasion than white gold. This means that enclosed diamonds or pearls sit firmly in the jewelry setting. The comparatively low density (D = 12.0) compared to gold (D = 19.3) and platinum (D = 21.5) also makes palladium attractive [29].
5.4 Dental alloys
Precious metal dealers offer special palladium alloys for dental technology in the form of small alloy chips in weight units of one gram or less. This allows crowns, large-span bridges, milling, cone and attachment work as well as model casting work to be produced, which can be veneered with suitable medium and high-fusing ceramics.
For example, a dental alloy consisting of 75 % palladium, 8.5 % silver, 6.0 % gold, 3.6 % tin and traces of gallium and ruthenium achieves a Vickers hardness of 250 and a density of only 11.6 g/cm³ with a white metal color [30].
Over 1400 dental alloys are approved in Germany [31]. These alloys, as well as dental solders and dental wires for braces, are subject to corresponding standards. The following apply to palladium alloys
- DIN EN ISO 8891 for alloys with a precious metal content of between 25 % and 75 %
- DIN EN ISO 969327 for alloys for the production of metal-ceramic (VMK) (bonding alloy)
- DIN EN ISO 15841 Dentistry - Wires for orthodontics
- DIN EN 29333 Dentistry - Brazing alloys and DIN EN ISO 9333 Dentistry - Dental brazing alloys.
5.5 Medical devices and instruments
In the highly technical and sensitive field of medical technology, only materials that can withstand a wide range of demands and stresses can be used. In many cases, this can only be demanded of precious metals. In addition to silver, gold and platinum, palladium is an indispensable raw material.
Palladium alloys can be found in pacemakers and defibrillators. Ultra-fine platinum metal wires, thinner than a human hair, are used to treat life-threatening aneurysms by stabilizing blood vessels and preventing cerebral haemorrhages [32].
Aerosol drug delivery with vibrating palladium meshes is an example of innovative, award-winning precision medical technology that has been successfully used in several million patients [33].
Many medical alloys have to take into account the individual body tolerances of patients. With titanium-palladium alloys, for example, this condition can be fulfilled [34].
Palladium is also used in sophisticated medical instruments and in the top layer coatings of implants.
5.6 Electrical and electronics industry
A significant number of electrical and electronic components contain palladium, its alloys or doublés.
For example, palladium is found in contact materials for relays in communication systems, in Pd/Ni alloys as a substitute for gold in the electrical industry, in electrode materials for fuel cells and spark plugs, in semiconductor components based on gallium nitride and in printed circuit board coatings.
Inside every smartphone there are various components containing precious metals, such as gold, silver and palladium. They are used both in the components and for the connections between the individual parts [35]. Palladium-containing components are also found in every PC, which, given the short range of this precious metal, requires largely quantitative recycling [36].
- to be continued -
Literature
[1] Weeks, M.E.: Discovery of the Elements, Journal of Chemical Education, 6th ed. (1956) 409
[2] https://de.wikipedia.org/wiki/(2)_Pallas
[3] Wollaston, W.H.: On a New Metal, Found in Crude Platina, Phil. Trans. R. Soc. 94 (1804) 419-430
[4] Graham, Th.: On the relation of hydrogen to palladium, Proceedings of the Royal Society of London. 17 (1869) 212-220
[5] Phillips, F.C., Am. Chem. J., 16 (1894) 255-277
[6] Edelmetall-Taschenbuch, Degussa (1967) 72
[7] http://forkat.anorg.chemie.tu-muenchen.de/biblio/texte/pdf
[8] Precious Metals Pocketbook, Degussa, Hüthig (1995)
[9] https://www.wotech-technical-media.de/womag/ausgabe/2013/10/21_w_edelmetalle_schade_10j2013/21.php
[10] Precious Metals Pocketbook, Degussa (1967) 70
[11] https://de.wikipedia.org/wiki/Platinmetalle/Tabellen_und_Grafiken
[12] https://de.wikipedia.org/wiki/Palladium
[13] Holleman; Wiberg: Inorganic Chemistry, vol. 2, 103rd ed. (2017) 2039
[14] https://de.wikipedia.org/wiki/Thioether
[15] https://www.kettner-edelmetalle.de/Palladium
[16] https://www.scheideanstalt.de/aktuelle-ankaufskurse/palladium/
[17] https://www.handelsblatt.com/finanzen/maerkte/devisen-rohstoffe/rohstoffe-warum-der-palladium-preis-seit-jahresbeginn-explodiert/
[18] https://feingoldhandel.de/palladium-das-silberweisse-edelmetall
[19] Aston, J.G.; Mitacek, P. Jr.: Structure of hydrides of palladium, Nature, London, UK, 195 (1962) 70-71
[20] https://www.pv-magazine.de/2017/09/01/solarstrom-wirtschaftlich-in-wasserstoff-umwandeln/
[21] https://www.seilnacht.com/Lexikon/swasser.html
[22] https://de.wikipedia.org/wiki/Anthrachinon-Verfahren
[23] Kickelbick, G.: Chemistry for Engineers, Pearson Germany, (2008) 155
[24] https://de.wikipedia.org/wiki/Herbert_Lindlar
[25] Rosenmund, K.W.: Über eine neue Methode zur Darstellung von Aldehyden, 1. Mitteilung, Berichte der deutschen chemischen Gesellschaft. 51 (1918) 585-593
[26] Steinborn, D.: Fundamentals of organometallic complex catalysis, Teubner, Wiesbaden (2007) 283-292
[27] https://www.chemhui.com/pro/37668
[28] Yang, J.; Liu, J.; Neumann, H.; Franke, R.; Jackstell, R.; Beller, M.: Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes, Science, 366 (2019) 1514
[29] https://goetze-gold.de/halbzeuge-zuschnitt/blech/platin-und-palladium/
[30] https://www.wegold.de/produkte/legierungen/palladium-basis-legierungen/676-wegold-dg-dentallegierung
[31] https://de.wikipedia.org/wiki/Dentallegierungen
[32] https://www.edelmetalle.org/medizintechnik/
[33] https://www.aerogen.com/de/technologie/
[34] https://patents.google.com/patent/DE60003490T2/de
[35] https://www.edelmetalle.org/elektronik/
[36] Buth, M.A.: Palladium from PC and electronics, special volume, adrenalinemedia (2012)
[37] RÖMPP, Lexikon Chemie, 8th edition, vol. 4 (1985) 2973
[38] https://de.wikipedia.org/wiki/Ilja_Iljitsch_Tschernjajew
[39] https://de.wikipedia.org/wiki/Trans-Effekt
[40] Tanaka, M.: Structure of Bis(tri-tert-butylphosphine)palladium(0), Acta Cryst., C48 (1992) 739-740
[41] He, L.-Y.: Bis(tri-tert-butylphosphine)palladium(0) [Pd(t-Bu3P)2], Synlett, 26/6 (2015) 551-852
[42] https://shop.jentner.de/galvanische-elektrolyte/edelmetall-elektrolyte/Palladiumbad/
[43] Talgner, F.; Myers, M.; Schmidt, H.: Galvanic silver-palladium as a contact surface, Galvanotechnik, Leuze Verlag, 05 (2017) 910
[44] Hasenpusch, W.: Explosivity of tetramine metal nitrates, J. prakt. Chem, Chemiker-Zeitung 335 (1993) 193-196
[45] Chemiker-Fachausschuss d. Gesellschaft " Metall und Erz": Analyse der Metalle I: Schiedsverfahren, Springer-Verlag, Berlin (1942) 184
[46] Burger,K.; Dyrssen, D.; Johansson, L.; Norén, B.; Munch-Petersen, J.: On the Complex Formation of Palladium with Dimethylglyoxime, Acta Chemica Scandinavica, 17 (1963) 1489
[47] Fries, J.; Getrost, H., Merck, E., Darmstadt: Organische Reagenzien für die Spurenanalyse (1975) 285
[48] https://en.wikipedia.org/wiki/Palladium(II)_bis(acetylacetonate)
[49] Setsune, J.: Palladium chemistry in recent porphyrin research Journal of Porphyrins and Phthalocyanines, 08/01(2004) 93-102
[50] To, W.; Liu, Y.; Lau, T.; Che, C.: A Robust Palladium(II)-Porphyrin Complex as Catalyst for Visible Light Induced Oxidative C-H Functionalization, Chemistry - A European Journal, 19/18 (2013)
[51] https://www.umweltbundesamt.de/platin-palladium#hinweise-zum-Recycling
[52] https://www.bmu.de/fileadmin/Daten_BMU/Pools/Forschungsdatenbank/fkz_3711_93_339_substitutionspotenziale_Metalle_bf.pdf
[53] Precious Metals Pocketbook, Degussa AG, Frankfurt, Hüthig- Verl. Heidelberg (1995) 48
[54] Doduco data book, Pforzheim (1974) 351
[55] Bertau, M.; Müller, A.; Fröhlich, P.; Katzberg, M.: Industrielle Anorganische Chemie, 4th edition, Wiley-Verlag, Weinheim (2013) 363-371
[56] Metal Research and Development, Degussa AG (1991) 33-44
[57] Annual Seminar Proceedings of the International Precious Metals Institute, IPMI, USA
[58] Knothe, M.; Hasenpusch, W.: The formation of explosive chlorine-nitrogen compounds during the processes of precious metals separation, Journal of Hazardous Materials, 56 (1997) 137-148
[59] https://de.wikipedia.org/wiki/Ameisensaeure
[60] https://capital.com/de/palladium-preisentwicklung
[61] https://www.boerseonline.de/nachrichten/rohstoffe/voruebergehende-flaute-warum-der-palladium-preis-bald-wieder-steigen-duerfte-102934254