The radioactive element thorium with its almost pure isotope Th-232 can look back on numerous applications. These include sputtering technology for surface finishing and the galvanic deposition of pure metal.
Due to the emitting alpha radiation, the more detailed risk assessment has decided against the further use of this element and its chemical compounds in most cases. However, thorium has a promising future with molten salt reactors for controlled nuclear energy production, which have been under development for several years. And perhaps modern safety precautions will also enable further applications of this abundant element.
The history of the discovery of thorium
As early as 1815, Berzelius had a sample of rock in which he suspected a new mineral. He assigned this mineral to a new oxide and named the corresponding metal after the Germanic god Thor. In 1824, however, it turned out that this supposedly new mineral was yttrium phosphate, YPO4[1].
In 1898, Marie Curie (1867-1934) and the German chemist Gerhard Schmidt (1865-1949) discovered the radioactivity of thorium at the same time [2, 3], with Gerhard Schmidt's publication appearing first [4].
But it was not until 1914 that D. Lely, Jr. and L. Hamburger succeeded for the first time in producing pure thorium metal using the method developed by the Dutch chemist Anton Eduard van Arkel (1893-1976) and the Dutch physicist Jan Hendrik De Boer (1899-1971) [5, 6].
Thorium metal and its properties
Thorium, named after the Germanic god Thor with the element symbol Th and atomic number 90, is in the periodic table of the elements in the actinide group, surrounded by the rare earth metals lanthanum (La), cerium (Ce) and praseodymium (Pr) and as neighbors of actinium (Ac) and protactinium (Pa) (Fig. 1). With its high atomic weight of 232.03 daltons, thorium emits alpha radiation with a half-life of 1.405 - 1010 years.
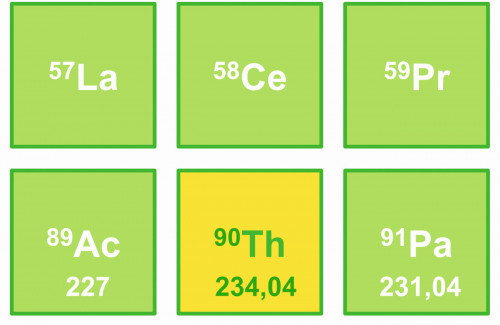 Fig. 1: Thorium and its neighbors in the periodic table of elements
Fig. 1: Thorium and its neighbors in the periodic table of elements
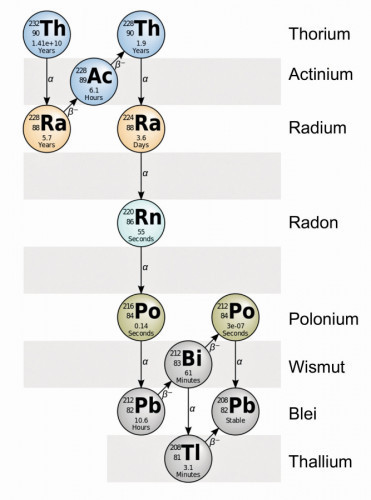 Fig. 2: 232Th decay series (source: https://en.wikipedia.org/wiki/Thorium)The thorium nucleus therefore consists of 90 protons and 142 neutrons orbiting 90 electrons in the configuration [He] 6d2 7d2 at different energy levels.
Fig. 2: 232Th decay series (source: https://en.wikipedia.org/wiki/Thorium)The thorium nucleus therefore consists of 90 protons and 142 neutrons orbiting 90 electrons in the configuration [He] 6d2 7d2 at different energy levels.
A long decay series begins with the element thorium and ends with the non-radioactive element lead (Fig. 2).
Pure thorium is a silvery-white metal that is stable in air at room temperature and retains its luster for several months. Metal oxidized on the surface slowly tarnishes in air and turns grey and eventually black.
The physical properties of thorium depend heavily on its purity. Many refined thorium metals contain traces of a few parts per thousand of thorium(IV) dioxide, ThO2. However, high-purity thorium can also be produced. It is soft, very ductile and can be cold rolled and drawn.
Thorium is polymorphic with two known modifications. At over 1400 °C, it transforms from a face-centered cubic structure to a body-centered cubic structure.
Thorium is only very slowly attacked by water, but it also dissolves only slowly in most dilute mineral acids, such as hydrofluoric acid, nitric acid or sulphuric acid, as well as concentrated hydrochloric and phosphoric acids. However, red fuming nitric acid, a mixture of 84 % nitric acid, HNO3, 13 % dinitrogen tetroxide, N2O4, and 3 % water, and "aqua regia", a mixture of three parts concentrated hydrochloric acid and one part concentrated nitric acid, dissolve thorium metal well.
When finely dispersed, powdered thorium has a pyrophoric effect, i.e. it burns in the air with a bright white flame. Further properties of the element can be seen in Figure 3.
Thorium deposits and prices
Thorium is found in the earth's crust with an abundance of 7 to 13 mg per kg, making it twice to three times more abundant than uranium. Due to its lithophilic character, the element is generally present in small quantities in almost all silicate rocks [7].
The concentration of thorium in seawater is orders of magnitude lower than that of uranium, making extraction from seawater uneconomical in any case.
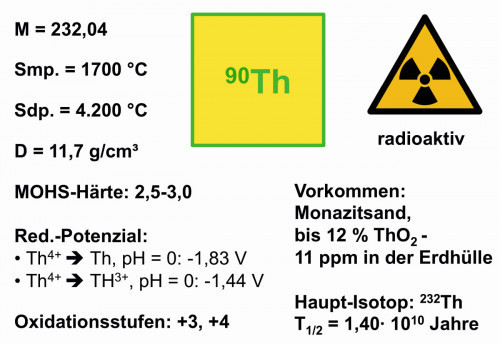 Fig. 3: Properties of the element thoriumThoriumis the 40th most abundant element and is one of the rarer elements on Earth, as it can be found widely distributed but is rarely found enriched in large quantities. In numerous minerals it is associated with uranium, lead or rare earth metals. Monazite sands with rare earths are the most important sources for thorium extraction.
Fig. 3: Properties of the element thoriumThoriumis the 40th most abundant element and is one of the rarer elements on Earth, as it can be found widely distributed but is rarely found enriched in large quantities. In numerous minerals it is associated with uranium, lead or rare earth metals. Monazite sands with rare earths are the most important sources for thorium extraction.
Monazite consists of around 60 % cerium phosphate, CePO4, and around 1 to 19 % thorium dioxide, ThO2. Thorium has been isolated from monazite for various purposes since the end of the 19th century.
The radioactive metal is mined in Australia, Norway, Sri Lanka, Canada, the USA, India, Lapland and Brazil. Silent deposits of around 800,000 tons are located in Turkey, mainly in the province of Eskişehir in the district of Sivrihisar.
Due to the known geochemistry of thorium, thorium deposits can also be expected in Germany. After all, there are numerous granitic rocks, gneisses and, in some places, reprocessed products of these rocks, which are found in sediments such as sandstones, near the surface or even exposed on the current land surface. In addition, the search for placer deposits of fluviatile origin could lead to thorium-containing monazite deposits.
The Geochemical Atlas of Saxony shows the state-wide distribution of numerous elements in the near-surface rocks and soils with exploitable thorium contents [8].
Between 2008 and 2017, the world market price for 1 kg of thorium was still US$ 60 [9, 10]; one year later, it was already listed at US$ 162 [11].
Ore processing and metal extraction
The thorium content of minerals in the earth's crust can be detected with sufficient accuracy at the earth's surface using gamma spectroscopy based on its specific gamma spectrum. This applies in any case from a content of one gram per ton of rock.
The most economical method for extracting thorium is digestion with hot sulphuric acid at over 200°C with subsequent hydrolytic precipitation by dilution with water. The slow dissolution of the mineral grains and the complexation of the dissolved metal ions by phosphates and sulphates and the associated different process parameters are problematic.
From around 1950, interest in higher purity thorium for use in nuclear processes increased. The sulphuric acid process was supplemented by precipitation with oxalates, which are converted to thorium hydroxide. As this product is still contaminated with traces of rare earth metals, it is then reacted with nitric acid to form soluble nitrates. With the aid of liquid-liquid extraction of thorium with tri-n-butyl phosphate, TBP/kerosene, analogous to the PUREX process, the work-up is carried out to pure thorium(IV) nitrate, Th(NO3)4 [12].
As thorium has a low electronegativity of 1.3 on the "Pauling scale", a direct reduction of its compounds cannot be carried out with the aid of carbon or hydrogen. Highly fusible thorium carbides or hydrides would be formed.
Three main processes are available for the production of pure thorium metal:
- The fusion electrolysis of thorium halidesin salt baths, usually using the variations KThF5 in NaCl, ThF4 in NaCl/ KCl or ThCl4 in NaCl/ KCl
- Reaction with base metals: ThO2 with Ca, ThCl4 with Mg or ThF4 with Ca
- The gas phase transport reaction, the thermal decomposition of ThI4 according to the Van Arkel de Boer process.
The powders or "metal sponges" obtained in this way are remelted into solid material under inert gas or in a vacuum [13].
Use of thorium
Due to radiation exposure, numerous applications of thorium and its compounds have been abandoned. These include the production of incandescent mantles, use as an X-ray contrast agent, the use of thorium dioxide for optical glasses with a high refractive index and the addition of 1 to 4 % thorium dioxide to Worfram inert gas welding electrodes to improve the ignition properties.
The construction of an "atomic nucleus clock" with excited LASER light using thorium-229 isotope, a research project funded by the European Research Council with around 14 million euros [14].
In recent years, the thorium molten salt reactor has emerged as the new beacon of hope for an enormously abundant and tamable energy generation process that could make us independent of fossil fuels of any kind.
Thorium reactors for energy generation
As American nuclear physicists have already been able to show in previous years, thorium can be used to produce the fissile uraniumisotope233Umuch more safely and easily than conventional uranium-plutonium mixed oxide (MOX) reactors.
Unlike in the uranium-plutonium breeder reactor, the fast breeder, this process is also possible in a reactor in which nuclear fission takes place using thermal neutrons (Fig. 4). This is due to the particularly high effective cross-section of 232Thfor the capture of a thermal neutron. Although the achievable hatching rates with such a thermal breeder with thorium are lower than with a fast breeder, it is much safer and more economical to operate.
 Fig. 4: The Chinese thorium reactor is due to go into series production in 2030 (Photo: Commons, Stern)
Fig. 4: The Chinese thorium reactor is due to go into series production in 2030 (Photo: Commons, Stern)
Through neutron irradiation, the reactor "breeds" thorium-233 from thorium-232, which is converted into uranium-233 via protactinium-233 with the corresponding half-lives by emitting nuclear electrons, the beta rays:
90Th-232+ 0n-1→ 90Th-233- e- → 91Pa-233- e → 92U-233.
Thorium with an atomic weight of 232 and 90 protons in its nucleus is bombarded with an additional neutron, which increases its atomic weight to 233. However, this releases an electron from its neutron inventory, which leads to protactinium, Pa, with 91 protons. This electron or β-ray emission takes place with a half-life of 22.3 minutes. The corresponding step to uranium-233 with 92 protons is significantly slower with a half-life of 26.967 days, i.e. almost a month.
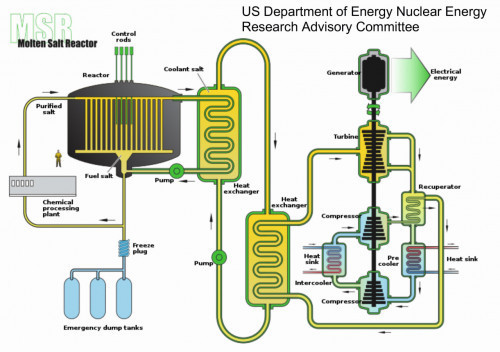
As thorium is available in larger quantities than uranium, it could potentially be an important source of energy in the future following the expected decline in global uranium reserves. In the early 2010s, there was an intensive campaign, particularly in the English-speaking world, for the use of thorium as a supposed solution to almost all energy problems [15]. Figure 5 shows the structure of a molten salt reactor with thorium as an energy source.
Recent studies also indicate that nuclear technology involving thorium poses considerable proliferation risks [16]. Access to nuclear bombs could be made easier for terrorists.
One current concept for a thorium-based thermal breeder reactor is the "molten salt reactor" [17], which is due to go into series production in China from 2030.
Research into the use of thorium in nuclear power plants is currently being carried out in India in particular, as this country has the world's largest thorium deposits [18].
Thorium compounds
In accordance with its position in the periodic table, thorium normally occurs in its compounds in the +4 oxidation state; thorium(III) and thorium(II) compounds are rarer.
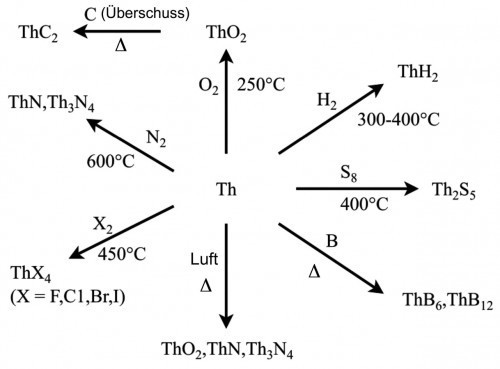
The most important compound is thorium dioxide, ThO2, which is commercially available at 6-8000 Euro/kg. Figure 6 shows the most important reactions for the production of preparations from the thorium metal.
Thorium dioxide
Thorium(IV) oxide or thorium dioxide, ThO2, is the only stable oxide of the radioactive element and actinide thorium, which occurs in nature as the mineral "thorianite". It is a colorless crystalline solid with the structure of calcium fluorite, CaF2.
It can be easily produced in pure form from thorium(IV) hydroxide, thorium(IV) oxalate, thorium(IV) carbonate or thorium(IV) nitrate by thermal decomposition. With a melting point of 3,390 °C, the compound has the highest melting point of all oxides. The density of
9.86 g/cm3 is accompanied by a high refractive index. Thorium dioxide is only soluble in strong acids and hardly soluble in water. The radioactivity of one gram of thorium(IV) oxide is 7,100 becquerels. This means that 7,100 alpha particles (helium nuclei) are emitted per second[19].
Thorium dichalcogenides
After thorium dioxide, the lilac-brown to purple-colored thorium disulfide, ThS2, is one of the thorium dichalcogenides. However, other sulfides of thorium are also known: the black thorium(II) sulfide, ThS, the brown thorium(III) sulfide, Th2S3, the black, Th7S12 (ThS1.71-ThS1.76), the deep red polysulfide Th3S7 and the thorium(IV) oxide sulfide, ThOS [20, 21].
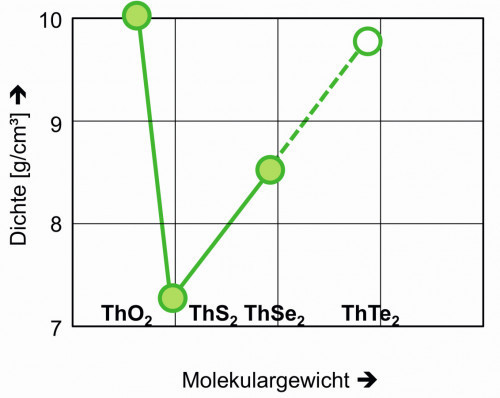
The thorium disulphide with a molecular weight of 296.17 g/mol, a decomposition temperature of about 1905 °C and a density of 7.36 g/cm3 at room temperature can be used as a solid lubricant [22-24].
Thorium(IV) selenide with a molecular weight of 389.96 g/mol and a density of 8.5 g/cm3 at room temperature is also available as a sputtering target for surface coatings [25].
Thorium(IV) telluride, M = 487.24, is also occasionally used for the same application. Its density was graphically estimated at 9.8 g/cm3 using Figure 7.
Thorium sulphates
Thorium(IV) sulphate, Th(SO4)2, is an easily water-soluble, colorless compound with a density of 4.255 g/cm3. From aqueous solution, the compound crystallizes in the form of hydrates, the water content of which depends on the conditions of presentation. The thorium sulphate tetrahydrate Th(SO4)2 - 4 H2Ocrystallizes in white needles. The sulphates with 8 and 9 water of crystallization molecules are also well studied. A solubility of 13.8 g/L is given for the sulphate with 9 H2Oat 20 °C. Anhydrous thorium(IV) sulphate is obtained by fuming thorium(IV) oxide with conc. sulphuric acid [26]. Thorium sulphate forms relatively poorly soluble sulphatothorates with alkali sulphates in water, such as Na2[Th(SO4)3] - n H2O, Na4[Th(SO4)4] - n H2Oor K6[Th(SO4)5] -n H2O[27].
Thorium nitride and nitrate
Thorium nitride, Th3N4, with a molecular weight of 752.14g/mol and a density of 11.6 g/cm3 only melts at 2,820 °C [26].
Thorium nitrate, Th(NO3)4, is a colorless chemical compound that is easily soluble in water and ethanol and is an important intermediate in the preparation of thorium(IV) oxide and thorium metal [27, 28]. With a molecular weight of 480.06 g/mol and a density of only 2.78 g/cm3, it decomposes at 50 °C, releasing nitrogen oxide gas and oxygen:
Th(NO3)4 → ThO2 + 4 NO2 + O2.
Thorium nitrate was once used in many ways: In the production of annealing dies, for TIG welding electrodes, cathodes for magnetron and traveling wave tubes, as a starting material for the toothpaste "Doramad", which was considered particularly healthy at the time, as well as bath additives and tinctures, which were supposed to help against eczema in particular [29].
Thorium halides
Thorium(IV) fluoride is a white solid in the form of a cryptocrystalline powder or single refractive, iridescent crystals. It is almost insoluble in water and has a monoclinic crystal structure. The compound reacts with atmospheric moisture at temperatures above 500 °C to form thorium oxyfluoride, ThOF2. When prepared from solutions, it is present in the form of hydrates, of which the hemihydrate is the only one of which the structure is known. The water of crystallization can be removed from the hemihydrate by heating in a vacuum to 400 °C [30].
Thorium(IV) fluoride is used to produce pure thorium and high-temperature ceramics. It is also used as a sputtering target for the production of thin films with a low refractive index without absorption in the visible and UV range. It is also used in the production of carbon arc lamps.
In addition, attempts are being made to use thorium(IV) fluoride as fuel for a liquid fluoride thorium reactor, LFTR. The first examples of these reactors have been under development in Japan since 2010 and in China since 2014 [31].
Thorium(IV) chloride can be obtained by chlorination of thorium(IV) oxalate with a mixture of carbon dioxide and carbon tetrachloride and by reaction of thorium(IV) oxide with carbon and chlorine or with carbon tetrachloride alone. The compound is a colorless, hygroscopic, crystalline solid that sublimates into large needles. In addition to an octahydrate, other hydrates of the compound are also known. Thorium(IV) chloride is used as an intermediate in the production of thorium [32].
Thorium(IV) bromide is produced by reacting thorium(IV) oxide with bromine and carbon at 800 to 900°C. The α-thorium(IV) bromide is formed when the mixture is heated to 330-375 °C for a longer period of time. Pure β-thorium(IV) bromide is formed when heated to 470 °C and quenched in ice water. Both modifications, of which hydrates are also known, form white, crystalline, hygroscopic compounds that are very soluble in water, ethanol and acetic ester [33].
Thorium(IV) iodide can be obtained by reacting thorium(IV) carbide or thorium with iodine at 500 °C. Furthermore, a synthesis with thorium(IV) hydride and hydrogen iodide is possible:
ThH4 + 4 HI → ThI4 + 2 H2.
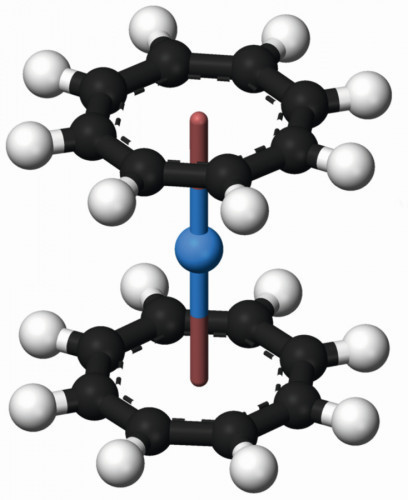 Fig. 9: Structure of thorocene, yellow cyclooctatetraenide complex, Th(C8H8)2Thorium(IV) iodide is an extremely air- and moisture-sensitive solid, which is present in the form of orange-colored crystal platelets in the heat and yellow in the cold. It serves as an intermediate product for the production of high-purity thorium by thermal decomposition of the compound according to the "Van Arkel de Boer process" [34].
Fig. 9: Structure of thorocene, yellow cyclooctatetraenide complex, Th(C8H8)2Thorium(IV) iodide is an extremely air- and moisture-sensitive solid, which is present in the form of orange-colored crystal platelets in the heat and yellow in the cold. It serves as an intermediate product for the production of high-purity thorium by thermal decomposition of the compound according to the "Van Arkel de Boer process" [34].
In addition, black thorium(II) iodide exists in two hexagonal modifications (M = 612.75; Smp. = 850 °C (Z.); D = 7.21 g/cm3) [35] as well as the black, violet-tinged, orthorhombic thorium(III) iodide (M = 612.75) [36].
For the analysis and separation of thorium from the rare earth metals, the thiosulphate method, the quantitative precipitation of thorium as thorium thiosulphate, Th(S2O3)2, from neutral chloride solutions and the iodate method, the quantitative precipitation as thorium iodate, Th(IO3)4, from strongly nitric acid solutions, should be mentioned [37]. Even in pure water, the iodate only dissolves to 0.369 g/L at 20 °C [38].
Complexes of thorium
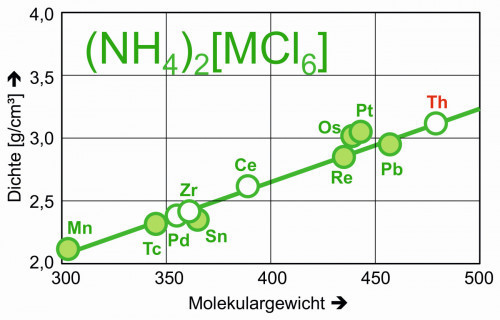
Like other tetravalent central atoms, thorium also forms halide complexes of the type [ThHal6]2-. The density of, for example, ammonium hexachloridothorate(IV), (NH4)2[ThCl6], can be estimated at 3.1 g/cm3 with the aid of analogous compounds (Fig. 8).
Most work on organothorium compounds concentrates on the sandwich structures of cyclopentadienyls and cyclooctatetraenyls (Fig. 9), which can be produced with thorium(IV) fluoride and the Grignard compounds of the ring compounds [18]. The thorium complex with "Arsenazo III", according to IUPAC: (3Z,6E)-3,6-bis[(2-arsenophyl)-hydrazinylidene]-4,5-dioxonaphthalene-2,7-disulfonic acid (Fig. 10) is used for both quantitative analysis and extraction [39]. The UV spectrum of the corresponding thorium complex (Fig. 11) allows the quantitative analysis of uranium, rare earths and a number of other metals [40].
Thorium in electroplating
The electrodeposition of thorium from salt solutions and molten salts is well known and has been described many times [41]. At least in the patent literature, the electroplating of thorium in combination with other metals appears again and again [42].
Trace metals such as thorium on graphite can also be easily separated analytically from ore deposit effluents and other wastewater and quantitatively determined via radioactivity [43].
The high thermodynamic stability of thorium has proven to be very advantageous for electroplating, especially in combination with precious metal alloys. Studies on solid palladium-thorium melts with up to 16 atomic % thorium in calcium fluoride electrolyte cells at 600 to 800 °C showed exceptional stability [44].
In the 1930s, the Fischer-Tropsch synthesis for the production of hydrocarbons from synthesis gas (CO/H2) originally used a cobalt-thorium catalyst [45], a process that could be used again today for sustainable galvanic applications.
Safety when handling thorium and its compounds
Thorium is an α-emitter and, as it decays, also an β-emitter and, due to these types of radiation, is dangerous if inhaled or ingested. Metal dusts and, above all, oxides are particularly risky radiotoxically due to their respirable nature and can cause cancer.
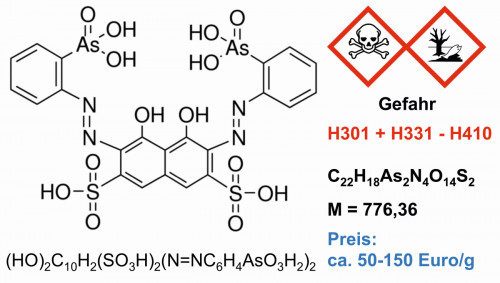 Fig. 11: UV spectra of complexing agent and thorium complex
Fig. 11: UV spectra of complexing agent and thorium complex
Classifications according to the CLP Regulation (Classification - Labelling - Packaging) [46] are not available because these only cover the chemical hazard, which plays a completely subordinate role in alpha emitters such as thorium and its compounds compared to radioactivity and its health consequences.
The acute chemical toxicity of thorium and its compounds is considered by toxicologists to be low and is essentially attributed to the radioactivity. This is due to the poor water solubility of 0.0001 μg per liter of the pure metal and the mostly occurring thorium dioxide. Thorium only dissolves in significant quantities in very acidic environments from a pH value < 4 and with complexing agents.
With a half-life of 14.05 billion years, the thorium isotope Th-232 is considerably less radioactive in its dose rate than uranium-238, as the longer half-life means that fewer decays occur per second and the concentration of short-lived decay products remains lower. As an α-emitter, the radiation has a short range, but the danger is when it enters the body.
When storing and handling thorium and its compounds, the permanent presence of elements from the decay series must also be taken into account. Particularly dangerous are the beta rays and the gamma rays, which have a high 2.6 MeV content and are very energetic and penetrative [18].
Literature
[1] Berzelius J. J.: Untersuchung eines neuen Minerals und einer darin enthaltenen zuvor unbekannten Erde, Annalen der Physik und Chemie, 92 (1829) 385-415
[2] Curie, M.: Rayons émis par les composés de l'uranium et du thorium, Comptes rendus de l'Académie des sciences, 126 (1898) 1101-1103
[3] Badash, L.: The discovery of thorium's radioactivity, Journal of Chemical Education, 43 (1966) 219-220
[4] https://de.wikipedia.org/wiki/Entdeckung_der_Radioaktivitaet
[5] Van Arkel, A.E; de Boer, J.H.: Darstellung von reinem Titanium-, Zirkonium-, Hafnium- und Thoriummetall, Z. anorg. allgem. Chem, 148/1 (1925) 345-350
[6] Lely, D. Jr.; Hamburger, L.: Herstellung der Elemente Thorium, Uranium, Zirkon und Titanium, Z. anorg. allgem. Chem., 87/1 (1914) 209-228
[7] Merkel, B.; Dudel, G. u. a.: Untersuchungen zur radiologischjen Emission des Uran-Tailings Schneckenstein, TU Bergakademie Freiburg und TU Dresden (1988)
[8] https://kipdf.com/thoriumlagerstaetten-weltweit-und-in-deutschland_5ac5f8141723dde15d5ac1bd.html
[9] https://www.final-frontier.ch/thoriumenergie
[10] https://www.mitsu-talk.de/forum/index.php?thread/57306-mit-8-gramm-100-jahre-fahren/
[11] http://www.leonland.de/elements_by_price/de/list
[12] https://de.wikipedia.org/wiki/PUREX-Prozess; PUREX = Plutonium-Uranium Recovery by Extraction
[13] Ullmann's Encyclopedia of industrial chemistry: Thorium, 8th ed. (2015)
[14] Seiferle, B. et al: Energy of the 229Th nuclear clock transition, Nature 573, (2019) 243-246
[15] Martin, R.: Super-Fuel: Thorium, the Green Energy Source for the Future, (2012)
[16] Thorium Report 2008, Oslo
[17] Ashley, S.F.; Parks, G.T.; Nuttall, W.J.; Boxall, C.; Grimes, R.W.: Thorium fuel has risks, Nature. Vol. 492/ 7427 (2012) 31-33
[18] https://de.wikipedia.org/wiki/Thorium
[19] https://de.wikipedia.org/wiki/Thorium(IV)-oxid
[20] https://wiki.edu.vn/wiki9/2020/12/12/verbindungen-von-thorium-wikipedia/
[21] Moscow Institute of Physics and Technology, MIPT (2020)
[22] Brauer, G. (ed.): Handbuch der Präparativen Anorganischen Chemie, 3rd, umarb. ed., vol. II. Enke, Stuttgart (1978) 1148
[23] Perry D.L.: Handbook of Inorganic Compounds, Second Ed, CRC Press (2011) 489
[24] https://de.wikipedia.org/wiki/Thorium(IV)-sulfide
[25] https://www.americanelements.com/thorium-selenide-60763-24-8
[26] https://www.spektrum.de/lexikon/chemie/thorium-iv-sulfat/9238
[27] Brown, D. ; Wiedemeyer, H.: Thorium: Compounds with S, Se, Te, B Gmelin Handbook, Springer (2013) 63
[28] https://en.wikipedia.org/wiki/Thorium_compounds
[29] https://de.wikipedia.org/wiki/Thoriumnitrat
[30] https://de.wikipedia.org/wiki/Thorium(IV)-fluoride
[31] Odenwald, M: Garant für die Weltenergieversorgung, Focus online, May 5 (2011) 3
[32] https://de.wikipedia.org/wiki/Thorium(IV)-chlorid
[33] https://de.wikipedia.org/wiki/Thorium(IV)-bromide
[34] https://de.wikipedia.org/wiki/Thorium(IV)-iodide
[35] https://de.wikipedia.org/wiki/Thorium(II)-iodide
[36] https://de.wikipedia.org/wiki/Thorium(III)-iodide
[47] Proske, O. et al: Analyse der Metalle I - Schiedsverfahren, Springer, Berlin (1942) 125-127
[38] https://en.wikipedia.org/wiki/Solubility_table
[39] Zeinab F. Hegazy, M.A.: Selective cloud point extraction of thorium (IV) using tetraazonium based ionic liquid, Journal of Environmental Chemical Engineering, Elsevier, 8/5 (2020)
[40] Fries, J. Getrost, H.: Organische Reagenzien für die Spurenanalyse, Merck, Darmstadt (1975) 352-353
[41] Müller, R.: Allgem. und techn. Elektrometallurgie, Springer, Wien (1932) 306
[42] https://patents.google.com/patent/DE1521063B2 (1974)
[43] https://www.dbu.de/OPAC/ab/DBU-Abschlussbericht-AZ-33285_01-Hauptbericht.pdf
[44] Schaller, H.-J.: Über die extrem hohe thermodynamische Stabilität von Pd-Th-Legierungen, Z. Naturforsch., 34 a (1979) 464-468
[45] https://de.wikipedia.org/wiki/Fischer-Tropsch-Synthese
[46] European GHS Regulation (EC) No. 1272/2008, called CLP Regulation (Classification, Labeling and Packaging), entered into force: January 20, 2009