At around 0.4 %, titanium is the tenth most common element in the earth's crust. It is mainly found there in the form of ilmenite, an iron titanate, and rutile, a calcium titanate. Extraction from seawater is less productive, with contents of between 1-2 mg titanium/m3. Titanium is extracted from ores in two stages: the first, the isolation of titanium dioxide using the sulphate and chlorination process, leads to the important commercial form of titanium, while in the second stage, titanium can be obtained by reduction using very base metals.
Corrosion resistance and low density favor titanium and its alloys for applications in the aerospace industry. Titanium dioxide is established as a white pigment in paints, plastics, ceramics and paper as well as a starting material for many titanium compounds. However, its use in cosmetic products and foodstuffs has suffered a setback.
Titanium, a robust light metal
The transition metal titanium with its many outstanding properties, such as its whitish metallic sheen, low density, high strength and corrosion resistance, is the 22nd element with an atomic weight of 47.87 g/mol in the 4th subgroup of the periodic table of elements. Titanium is surrounded in the 4th period by scandium and vanadium as well as the heavier elements yttrium, zirconium and niobium. The 4th subgroup continues via zirconium to hafnium.
The odd atomic weight of 47.87 is due to the atomic weights of the mixture of five naturally occurring, stable titanium isotopes:
46 Ti : 8.0 %
47 Ti : 7.3 %
48 Ti : 73.8 %
49 Ti : 5.5 %
50 Ti : 5.4 %.
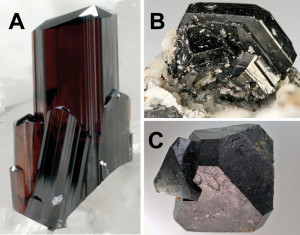 Figure 2: Rutile, TiO2 (A), ilmenite, FeTiO3 (B) and perovskite, CaTiO3 (C) The normal potential to reduce titanium dioxide to titanium is -0.86 volts, the electronegativity according to L. Pauling is 1.54. Titanium occurs in its compounds +2-, +3- and +4-valent.
Figure 2: Rutile, TiO2 (A), ilmenite, FeTiO3 (B) and perovskite, CaTiO3 (C) The normal potential to reduce titanium dioxide to titanium is -0.86 volts, the electronegativity according to L. Pauling is 1.54. Titanium occurs in its compounds +2-, +3- and +4-valent.
The light metal is therefore particularly suitable for applications where high corrosion resistance, strength and low weight are important. Due to the complicated manufacturing process, titanium is ten times more expensive than conventional steel [1].
Titanium forms an extremely resistant oxidic protective layer in air, which makes it corrosion-resistant in many media. Above a temperature of 400 °C, however, the strength properties quickly decline. High-purity titanium is ductile. At higher temperatures, it becomes brittle very quickly due to the absorption of oxygen, nitrogen and hydrogen. The high reactivity of titanium with many media at elevated temperatures or increased pressure must also be taken into account if the passive layer cannot withstand the chemical attack. This can lead to an explosive increase in reaction speed. At an oxygen pressure of 25 bar, titanium burns completely to form titanium dioxide in a highly exothermic reaction. Despite the passivation layer, it reacts with oxygen at temperatures above 880 °C and with chlorine at temperatures of 550 °C and above. Titanium also reacts exothermically with pure nitrogen, which must be taken into account, for example, when machining under N2 shielding gas due to the heat generated.
Titanium is resistant to dilute sulphuric acid, hydrochloric acid, chloride-containing solutions, cold nitric acid and most organic acids and alkalis such as sodium hydroxide. In concentrated sulphuric acid, however, it dissolves slowly, forming the violet titanium sulphate. Further properties of titanium can be found in footnote [2]. Titanium already combines with hydrogen at room temperature, at higher temperatures of 300 to 400 °C almost to TiH2. Twice as much hydrogen is stored in this compound as in the same volume of liquid hydrogen. Complete hydrogen release from the titanium hydride can only be expected at 1000 °C.
Discovery and presentation of titanium
 Fig. 3: Titanium analysis as an orange-colored peroxide complexTheelement titanium was discovered in 1791 by the English clergyman and enthusiastic mineralogist William Gregor (1761-1817) from Cornwall through remarkably accurate analyses of titanium iron mineral [3].
Fig. 3: Titanium analysis as an orange-colored peroxide complexTheelement titanium was discovered in 1791 by the English clergyman and enthusiastic mineralogist William Gregor (1761-1817) from Cornwall through remarkably accurate analyses of titanium iron mineral [3].
Four years later, the Berlin chemist Martin Heinrich Klaproth (1743-1817) came across the element while investigating rutile ore. He gave the element its current name based on the Greek gods of the Titans [4].
It was not until 1831 that the chemistry professor Justus von Liebig (1803-1873) succeeded in extracting the metallic titanium from the titanium ore in his Giessen laboratory [5].
In 1910, the American metallurgist Matthew Albert Hunter (1878-1961) produced titanium metal with a purity of 99.9% by heating titanium tetrachloride, TiCl4, with sodium to 700 to 800°C in a steel bomb.
Another 30 years were to pass before the Luxembourg metallurgist William Justin Kroll (1889-1973) made the industrial production of titanium metal in two steps possible in 1940 with a new patented process, the "Kroll process". First, titanium tetrachloride was produced, followed by reduction with magnesium.
Titanium(IV) oxide is converted to titanium(IV) chloride at temperatures of 750 to 1000 °C as part of a reducing chlorination process with chlorine and coke:
TiO2 + 2 Cl + 2 C → TiCl4 + 2 CO <1>
After fractional distillation of the liquid titanium tetrachloride at room temperature with a boiling point of 136.4 °C to separate the impurities from the ore, the reduction with magnesium takes place at temperatures of approx. 800-900 °C under an argon protective gas atmosphere to produce metallic titanium [6]:
TiCl4 + 2 Mg → Ti + 2 MgCl2 <2>
 Fig. 4: Titanium as a top material in aerospace technology, e.g: Lockheed SR-71, "Blackbird" In a vacuum distillation, the remaining magnesium escapes as well as like magnesium chloride with boiling points under normal conditions of 1,110 and 1,412 °C. The resulting titanium sponge (Fig. 1) with a purity of 99.1 to 99.7 % by weight and an oxygen content of between 0.06 and 0.3 % by weight is marketed as a crushed powder or as molten metal [7].
Fig. 4: Titanium as a top material in aerospace technology, e.g: Lockheed SR-71, "Blackbird" In a vacuum distillation, the remaining magnesium escapes as well as like magnesium chloride with boiling points under normal conditions of 1,110 and 1,412 °C. The resulting titanium sponge (Fig. 1) with a purity of 99.1 to 99.7 % by weight and an oxygen content of between 0.06 and 0.3 % by weight is marketed as a crushed powder or as molten metal [7].
Titanium sponge production is the basis of the titanium industry, but is an intermediate product that is mainly used for the production of titanium metal or titanium alloy ingots, which in turn are used for the production of ingots, tubes, bars, plates, sheets and other semi-finished titanium products.
The purest titanium can be obtained using the "Van Arkel de Boer process". The transport reaction process developed by the Dutch chemists Anton Eduard van Arkel (1893-1976) and Jan Hendrik de Boer (199-1971) in 1924 for the extraction and purification of metals such as titanium and other metals is based on a reaction with iodine at around 600 °C and thermal decomposition on tungsten wire at 1,200 °C [8]:
Ti + 2 I2 → TiI4 → Ti + 2 I2 <3>
In 2008, a ton of titanium sponge cost an average of 12,000 euros. In 2021, offers from China are around 8,000 euros [9].
Titanium deposits
As a relatively base element, titanium is only found in the earth's crust in compounds as an oxide, TiO2, or with other metals as titanate(IV). Although it only occurs in low concentrations, it is by no means rare, as it is the 10th most abundant element in the earth's crust with a content of 0.4 %. Significant mineral deposits are rutile, TiO2, ilmenite, FeTiO3, perovskite, CaTiO3(Fig. 2) and other metal titanates(IV), such as barium titanate, BaTiO3 and titanite, CaTiO(SiO4).
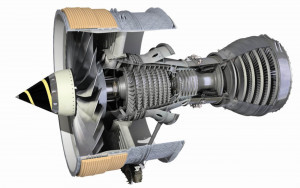 Fig. 5: Aircraft engine fan (turbofan) Trent 800, Rolls-Royce plc, with twenty-six blades made of the alloy Ti-6Al-4VAsof 2019, the mineral rutilealonewas found at 5900 locations worldwide [10]. Global mining reserves for the most important titanium minerals ilmenite and rutile were estimated at 692.58 million tons in 2014, with the largest regional shares falling to China with 28.9%, Australia with 17.0% and India with 13.3%.
Fig. 5: Aircraft engine fan (turbofan) Trent 800, Rolls-Royce plc, with twenty-six blades made of the alloy Ti-6Al-4VAsof 2019, the mineral rutilealonewas found at 5900 locations worldwide [10]. Global mining reserves for the most important titanium minerals ilmenite and rutile were estimated at 692.58 million tons in 2014, with the largest regional shares falling to China with 28.9%, Australia with 17.0% and India with 13.3%.
Other titanium deposits are located in Scandinavia, North America, the Urals and Malaysia. In 2010, deposits were discovered in Paraguay, the exploitation of which is still in the planning phase.
Titanium has also been detected in meteorites. Rock samples from the Apollo 17 moon mission contained up to 12.1% titanium dioxide, TiO2, which has already prompted thoughts of "asteroid mining" [1].
Plants contain around 1 mg titanium/kg dry matter and the human body contains an average of 15 mg, mainly in the lungs [11].
In Germany, for example, rutile has been detected in some regions of the Black Forest, in the Fichtelgebirge, Spessart, Bavarian Forest and Upper PalatinateForest, Hesse, Lower Saxony, the Siebengebirge in North Rhine-Westphalia, the Eifel, Saarland, the Saxon Ore Mountains, Schleswig-Holstein and Thuringia [1].
Analysis of titanium
To determine larger quantities of titanium, the element, which is usually tetravalent, is reduced to the trivalent oxidation state with zinc or aluminum. Titration against oxidizing agent standards, such as iron(III) ammonium sulfate, Fe(NH4)(SO4)2 - 12 H2O, or cerium sulfate, Ce(SO4)2 - 4 H2O, is thus possible.
Titanium traces can preferably be determined by spectrophotometry: By adding hydrogen peroxide in acidic solution [2], a yellow peroxytitanium complex is obtained (Fig. 3). Tiron (1,2-dihydroxybenzene-3,5-disulfonate), which is measured at 410 nm, also yields a yellow complex [12,13].
Today, titanium analyses are preferably analyzed using phycal determination methods, such as X-ray fluorescence, emission spectrometry or inductively coupled plasma atomic emission spectroscopy (ICP).
Titanium alloys
A number of standardized titanium alloys are available both in the form of light metal alloys and as steel coatings.
According to the US standard ASTM (American Society for Testing and Materials), there are 35 quality classes for titanium and its alloys, whereby the first four are reserved for pure titanium. They differ in terms of purity, corrosion resistance, ductility and strength. Grade 1 has the highest corrosion resistance and formability, but the lowest strength, while technically pure titanium of grade 4 has the highest strength [14]. The European material number for pure titanium is 3.7034. The most economically significant titanium alloys with 6 wt.% aluminum and 4 % vanadium are suitable for industrial applications under the material numbers 3.7165 and 3.7164 for aerospace applications (ASTM grade 5) (Fig. 4 and 5) [15, 16].
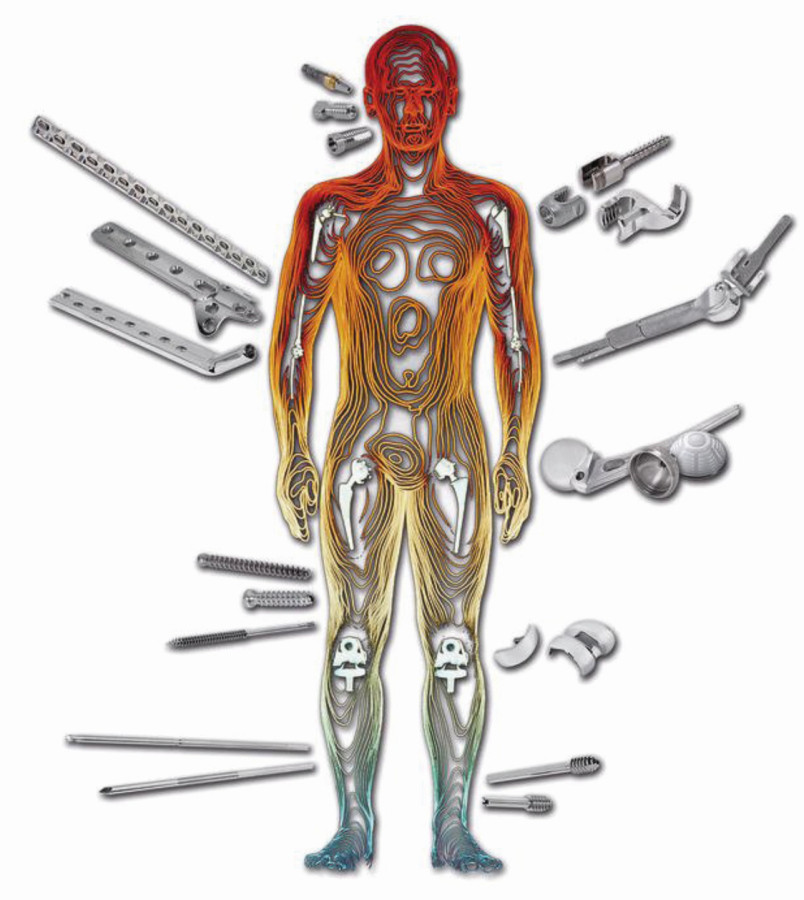 Fig. 6: Spare parts made of titanium or titanium alloys in medical technology
Fig. 6: Spare parts made of titanium or titanium alloys in medical technology
Alloys made of nickel (face-centered cubic) and titanium (hexagonal) belong to the "shape memory alloys" or "memory metals" with multiple applications, as they return to their original state after mechanical deformation due to their different crystal structures [17].
When added to steel in trace amounts, titanium gives the steel increased toughness, strength and ductility and prevents intercrystalline corrosion even in concentrations of 0.01 to 0.1 percent by weight.Titanium-based alloys with prices of around 45 euros/kg meet the highest requirements for applications in chloride-containing media and seawater.
Due to their low density, titanium alloys can also be found in many outdoor and sports articles, such as racing bikes, golf clubs, tennis rackets, diving knives, mountaineering screws and tent pegs.
Medical technology also likes to use titanium and its alloys, as they combine maximum biocompatibility, mechanical strength and corrosion resistance and are therefore ideal for stressed medical components, especially for load-bearing orthopaedic implants and dental materials (Fig. 6). Fine, spherical titanium powders of available materials, such as titanium of commercial purity or the alloys Ti-6Al-4V and Ti-6Al-7Nb [18, 19], are generally used as starting materials.
Titanium compounds
Of the titanium compounds [20], the polymorphic oxides are the most important, followed by the halides, especially the tetrachloride, which also serves as a starting material for metal production.
Titanium oxides
Titanium forms a number of different oxides [21, 22], of which the polymorphic titanium dioxide, TiO2, with its modifications rutile, anatase and brookite is the most important. In addition, a series of non-stoichiometric titanium suboxides of the type TinO2n-1(4 ≤ n ≤ 10) as well as titanium(III) oxide and titanium(II) oxide can be produced.
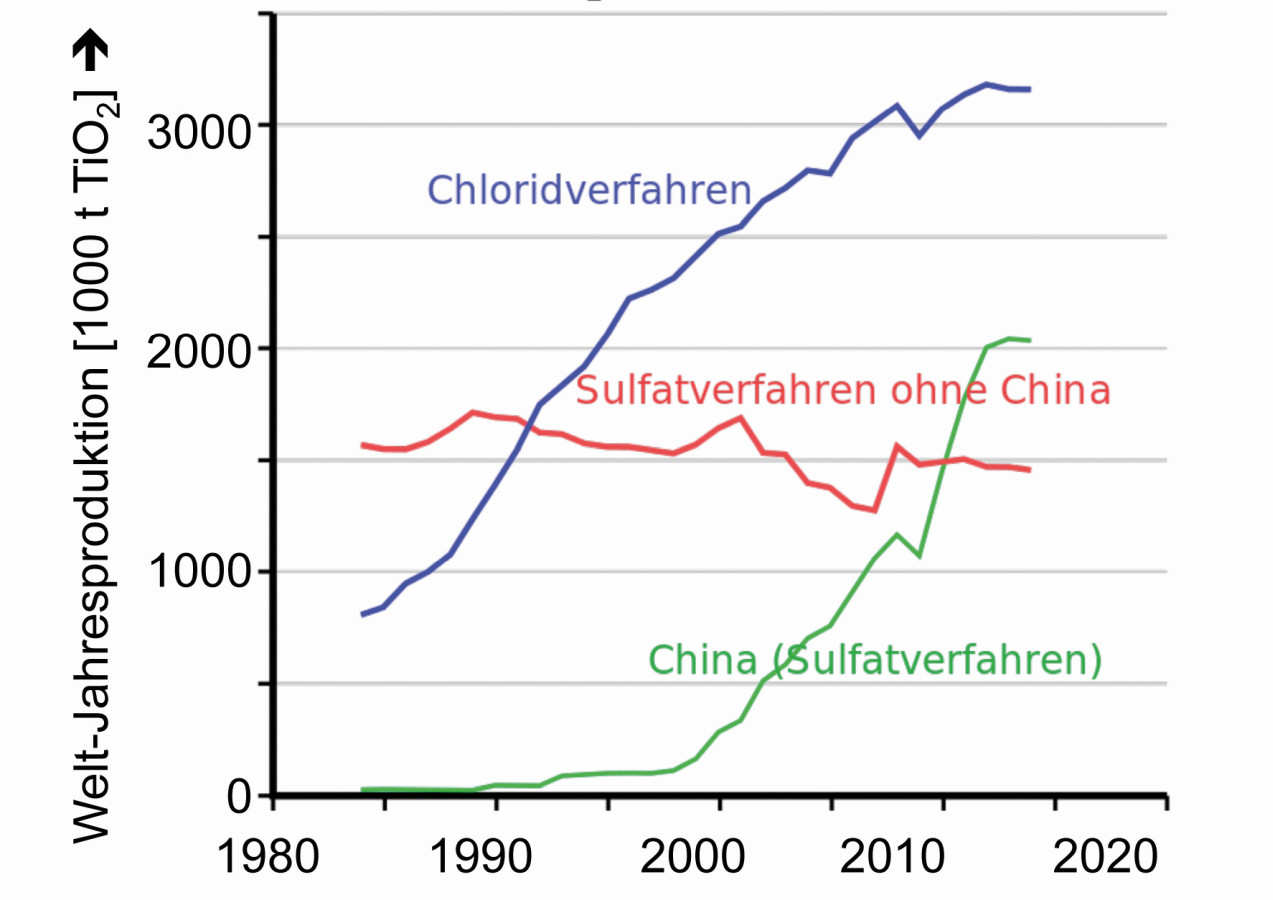 Fig. 7: Annual global production of titanium dioxide according to the different process variants
Fig. 7: Annual global production of titanium dioxide according to the different process variants
The golden-yellow, current-conducting titanium(II) oxide, TiO, exists in the range from Ti0.64Oto Ti1.26Oin the crystal structure of sodium chloride.
The dark purple titanium(III) oxide, Ti2O3, is found in the range from TiO1.49 to TiO1.51 with a trigonal crystal structure similar to corundum, Al2O3.
The dark blue titanium pentoxide, Ti3O5, is present up to 120 °C in the monoclinic crystal structure, above that in the orthorhombic modification. Above 175 °C, the compound changes from a semiconductor to a metallic conductor.
The oxides of the composition Ti4O7 to Ti10O19 or TiO1.75-TiO1.90, exhibit complex links of TiO6 octahedrons in their triclinic crystal structures.
Titanium dioxide, TiO2, occurs as a colorless compound in nature as rutile, anatase and brookite. In addition, eight other synthetically produced TiO2 modifications are known.
Titanium dioxide
In addition to the polymorphic oxides, titanium dioxide, TiO2, mainly in the rutile modification, is the most important titanium compound.
The melting point of titanium dioxide is 1855°C. The compound is thermally and chemically stable. It is light-resistant, inexpensive and therefore the most important white pigment. It is approved for use in food as additive E171 [23].
In 1980, 2.5 million tons of titanium dioxide came onto the world market, in 2018 it was already around 7 million tons (Fig. 7).
Two main processes are available for the production of pure titanium dioxide: the sulphate and chloride processes. A special route leads to TiO2 nanopowder (Fig. 8).
Sulphate process for TiO2 production
 Fig. 8: Titanium dioxide, the whitest white, as a powder (top left) and as a nanomaterial SEM (bottom right)The sulphate process for producing titanium dioxide was developed by the Norwegians F. Farup and G. Jebsen in 1915. It has been used commercially since 1916 and is still important today [24].
Fig. 8: Titanium dioxide, the whitest white, as a powder (top left) and as a nanomaterial SEM (bottom right)The sulphate process for producing titanium dioxide was developed by the Norwegians F. Farup and G. Jebsen in 1915. It has been used commercially since 1916 and is still important today [24].
The finely ground and enriched titanium iron ore, FeTiO3, (ilmenite) is digested with concentrated sulphuric acid to form iron sulphate, FeSO4 - 7 H2O, and titanium oxide sulphate, TiOSO4. As the iron is partly trivalent as Fe3+, the digestion solution must be mixed with pure iron scrap as a reducing agent in order to obtain only bivalent iron Fe2+. This can then be crystallized out of the solution as so-called "green salt", whereby the solubility in water is still relatively high. The density of the iron(II) sulphate decreases in relation to the molecular weight with increasing amounts of water of crystallization from 2.84 to 1.89 g/cm3 (20 °C) [25].
The titanium oxide sulphate remaining in solution precipitates as sparingly soluble titanium oxide hydrate through hydrolysis with water vapor. After several filtration and washing processes, the titanium oxide hydrate must be annealed at 800-950°C to obtain titanium dioxide.
By adding seed crystals and through the temperature, the modification of the TiO2 to be produced can be controlled in this step. The sulphate process is suitable for the production of both the anatase and rutile modification, while the chloride process is generally only used for the production of pure rutile.
The resulting by-products, the "dilute acid" with sulphuric acid and iron sulphate, were increasingly processed using a recycling process developed by Bayer AG in 1958. A 96 % sulphuric acid and crystallized "green salt" are available again, which can be thermally decomposed to iron oxide, Fe2O3[26].
Chloride process for TiO2 production
In the chloride process [27], predominantly rutile ore or residues with contents of preferably 94 to 96 % TiO2 are reacted with petroleum coke and chlorine at 800 to 1200 °C in a fluidized bed reactor to produce titanium tetrachloride, TiCl4[28].
However, poorer titanium slags are now also used.
After the titanium tetrachloride has been purified by distillation, the TiCl4 vapor is burned with oxygen to form titanium dioxide and chlorine gas at temperatures of 1000 to 1400 °C:
TiCl4 + O2 → TiO2 + 2 Cl2. <4>
The chlorine gas is again used to produce titanium tetrachloride, which is also used to produce titanium metal after various processes.
Figure 9 compares the two titanium dioxide production processes, the sulphate and the chloride process, once again in the flow chart.
Due to the increased demand for white pigments for paints and cosmetics, the chloride variant has established itself as the favored process in Germany, while the sulfate process is mainly used in China.
If titanium dioxide is compared with other analogous metal dioxides in the form of a density-molecular weight relation, a linear relationship D = 0.038 M + 1.0 results, so that an extrapolation to the density of rutherfordium dioxide, RfO2, with D = 12.5 g/cm3 also appears possible.
Titanium dioxide as a white pigment is used in large quantities for paint production. The refractive indices are interesting, reaching a relatively high value of n = 4.0 for titanium dioxide. Although the refractive index is even higher for pigments containing barium and lead, the toxic values are also in questionable ranges. Zinc oxide and calcium carbonate stand out as alternatives to titanium dioxide.
Concerns about titanium dioxide
Titanium dioxide or titanium (IV) oxide is widely used as a "whitening agent", both as a pigment in paints and in industrially produced foods and cosmetics.
On food packaging, titanium dioxide can be recognized under the E number 171, in cosmetics it is listed under the designation CI 77891. In 2013 alone, around 6.5 million tons of TiO2 were produced for these applications. However, despite its widespread use, titanium dioxide is not always harmless to health.
Several studies cast doubt on the safety of titanium oxide. For example, a large-scale study conducted at the University of Zurich in 2017 came to the conclusion that titanium dioxide could increase or even promote intestinal inflammation.
Fears even go so far as to suggest that inhaling titanium dioxide particles, especially in the nanometer range, can be carcinogenic. According to the Federal Institute for Risk Assessment, BfR, the EU Commission has decided to classify titanium dioxide as a hazardous substance with the indication "probably carcinogenic by inhalation". The risk mainly concerns cosmetic sprays, aerosols and powders, but not products containing the whitening agent TiO2 in solid form.
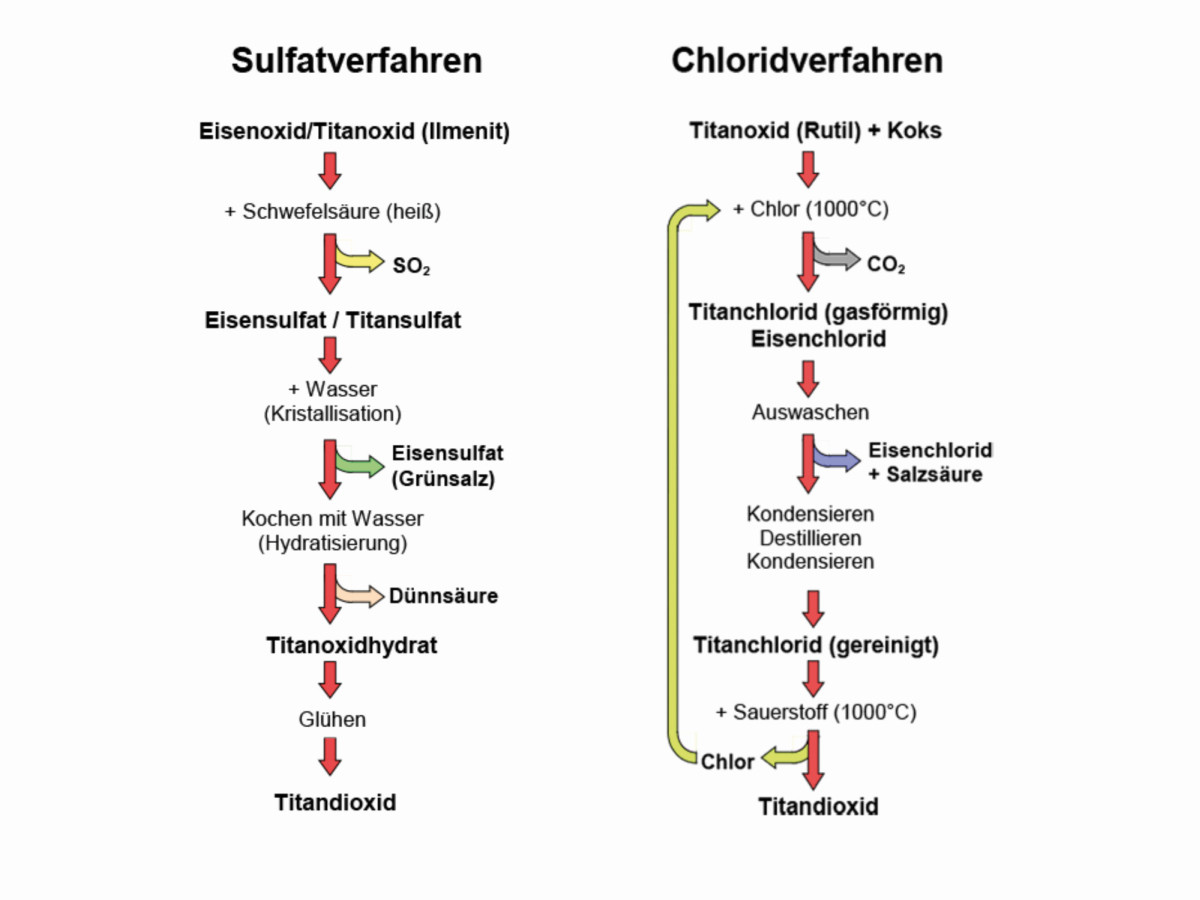 Fig. 9: Process for the production of titanium dioxide
Fig. 9: Process for the production of titanium dioxide
Due to the uncertain facts, France was the first European country to enforce a ban on titanium dioxide in food. The Netherlands intend to follow suit.
In Germany, the recommendations of the European Food Safety Authority (EFSA) are being followed. In May 2021, it stated in an updated assessment that titanium dioxide "can no longer be considered safe as a food additive". Further research will have to address these suspected risks.
The food company Dr. Oetker has not used titanium dioxide in its products since April 2020, after "foodwatch" launched a protest campaign that was signed by around 40,000 people [29].
Metal(II) titanates
Titanates are, for example, the salts of titanic acids, H2TiO3, which also occur in nature as minerals alongside orthotitanates after the formal orthotitanic acid, H4TiO4. They can also be regarded as mixed oxides of the type Me(II)O - TiO2.
Titanates are used as a ceramic material for piezo elements and ceramic capacitors. Many form ion crystals of perovskite structure with ferroelectric properties.
Natural titanates include the titaniumminerals perovskite, CaTiO3, barioperovskite, BaTiO3, macedonite, PbTiO3, ilmenite, FeTiO3, geikielite, MgTiO3, pyrophanite, MnTiO3, tausonite, SrTiO3[30].
Barium titanate, BaTiO3, and lead titanate, PbTiO3, are white or yellow powders and are also used as pigments in addition to utilizing their ferroelectric properties. Strontium titanate, SrTiO3, is a ceramic compound with very special properties in the form of plastic behavior. They are used as superconductors, but also as gemstones.
There is also a range of organic titanates. Examples include tetramethyl titanate, (CH3O)4Ti, tetrabutyl titanate, (H9C4O)4Ti, [31] tetraisopropyl titanate, (H3C-CHO-CH3)4Ti, tetraphenyl titanate, (Ph-O)4Ti, [32] or tetrahexyl titanate, (H13C6O)4Ti.
Titanium halides
The halides of titanium [33] exist in the 2-, 3- and 4-valent oxidation state of the type Ti(Hal)2, Ti(Hal)3 and Ti(Hal)4. Table 1 compares the properties of the titanium halides. The halides of bivalent, trivalent and tetravalent titanium show analogous curves, particularly in the density of the compounds in relation to the molecular weight.
|
Halide |
Color |
Formula |
M |
Smp. [°C] |
Sdp. [°C] |
D [g/cm3] |
H-phrases |
|
Difluoride |
TiF2 |
85,86 |
3,44 |
314 |
|||
|
dichloride |
black |
TiCl2 |
118,79 |
1035 |
1500 |
3,13 |
250-314-EUH 014 |
|
Dibromide |
black |
TiBr2 |
207,68 |
500 (Z.) |
4,41 |
none |
|
|
Diiodide |
black |
TiI2 |
301,68 |
480 (Z.) |
5,2 |
none |
|
|
trifluoride |
blue |
TiF3 |
104,88 |
1200 |
1400 |
3,4 |
314 |
|
trichloride |
violet |
TiCl3 |
154,26 |
440 (Z.) |
2,6 |
250-290-414-EUH 014 |
|
|
tribromide |
black-blue |
TiBr3 |
287,58 |
550 (Z.) |
4,24 |
none |
|
|
triiodide |
violet-black |
TiI3 |
428,60 |
310 (Z.) |
4,9 |
none |
|
|
tetrafluoride |
colorless |
TiF4 |
123,86 |
284 (subl.) |
2,8 |
302-312-332-314 |
|
|
Tetrachloride |
colorless |
TiCl4 |
189,71 |
-24,1 |
136,4 |
1,73 |
314-330-335-EUH 014 |
|
Tetrabromide |
yellow |
TiBr4 |
367,48 |
38-40 |
230 |
3,25 |
314 |
|
Tetraiodide |
red-brown |
TiI4 |
555,49 |
150 |
377 |
4,3 |
314 |
Titanium tetrachloride is used several times as an important Lewis acid in organic chemistry, for example in "Knoevenagel reactions" or the "Mukaiyama-Michael reaction". It is also a basic material for catalysts, such as "Ziegler-Natta catalysts", and a starting material for organo-titanium compounds.
Titanium tetrachloride is used in smoke generators for military purposes as a fog warfare agent and also in the production of titanium mirrors.
TiCl4 occurs as an intermediate product in the purification of titanium dioxide and in the production of titanium using the "Kroll process".
Other titanium compounds
Titanium compounds with boron, TiB, carbon, TiC, or nitrogen, TiN, are used as hard materials.
Titanium compounds are also used in the production of "cermets", which are composite materials made of ceramic and metal.
Cermets are distinguished from sintered carbides by their lack of electrical conductivity and their higher resistance to thermal shock and oxidation [34].
Titanium also forms interesting compounds with the chalcogenides sulphur, selenium and tellurium: The green titanium disulphide with a CdI2 layer structure and a comparatively low density of only 3.22 g/cm3 is used, for example, as a lubricant and electrode material for batteries and accumulators [35].
The gray titanium diselenide, TiSe2, with a higher density of 5.26 g/cm3 but also a cadmium iodide layer structure, also has application potential for electricity storage [36].
The semiconductor properties of the black, hexagonal titanium ditelluride, TiTe2, with a density of 6.34 g/cm3 can be utilized [37].
In terms of the densities of the titanium chalcogenides in relation to their molecular weights, the disulphide is characterized by a comparatively low density. With linear extrapolation, the corresponding titanium-polonium compound would have a density of around 7.8 g/cm3.
Finally, the titanium sapphires used for LASER, in which a few hundredths to tenths of a percent by weight of the Al3+ ions are replaced by Ti3+ ions with the formula of approximately Al2O3:Ti3+ by doping corundum while approximately retaining the density of 3.98 g/cm3. This produces a bright red sapphire, the active medium of the titanium-sapphire laser. It absorbs strongly in the 400 to 600 nm wavelength range with a maximum at 490 nm and fluoresces from 670 to 1070 nm with a maximum at 800 nm. In addition, the titanium sapphire has a high thermal conductivity, which allows the pump beam to be focused on a small area without destroying the crystal [38].
Literature
[1] https://institut-seltene-erden.de/seltene-erden-und-metalle/strategische-metalle-2/titan/
[2] https://de.wikipedia.org/wiki/Titan_(element)
[3] https://en.wikipedia.org/wiki/William_Gregor
[4] https://de.wikipedia.org/wiki/Martin_Heinrich_Klaproth
[5] https://de.wikipedia.org/wiki/Justus_von_Liebig
[6] https://de.wikipedia.org/wiki/Kroll-Prozess
[7] https://lkalloy.com/de/what-is-titanium-sponge?
[8] https://de.wikipedia.org/wiki/Van-Arkel-de-Boer-Verfahren
[9] https://german.alibaba.com/Popular/CN_titanium-priceper-ton-Trade.html
[10] https://de.wikipedia.org/wiki/Rutil
[11] Merian, E.: Metalle in der Umwelt, Verl. Chemie, Weinheim (1984) 585-588
[12] Binghua, Y. et al: Fabrication of Tiron-TiO2 chargetransfer complex with excellent visible-light photocatalytic performance, Materials Chemistry and Physics, 184, 1 (2016) 298-305
[13] https://de.wikipedia.org/wiki/Tiron
[14] https://fwmetals.de/materials/titanium/cp-titanium/
[15] https://www.tag.it/de/titanlegierungen-einfuehrungeigenschaften- and-applications/
[16] https://www.tag.it/de/titanlegierungen-dieklassifizierung-teil-2/
[17] https://de.wikipedia.org/wiki/Formgedaechtnislegierung
[18] https://www.ifam.fraunhofer.de/content/dam/ifam/de/documents/shaping_functional_materials/powder_technology/titan_fraunhofer_ifam.pdf
[19] https://mav.industrie.de/fertigung/werkzeuge/diebearbeitung-von-titan-in-der-medizinindustrie/#sliderintro-1
[20] https://de.wikipedia.org/wiki/Kategorie:Titanverbindung
[21] https://de.wikipedia.org/wiki/Titanoxide
[22] Cardarelli, F.: Materials Handbook: A Concise Desktop Reference, Springer Verl. Berlin, (2008) 617
[23] https://de.wikipedia.org/wiki/Titan(IV) oxide
[24] https://de.wikipedia.org/wiki/Sulfatverfahren_(titanium dioxide)
[25] https://de.wikipedia.org/wiki/Eisen(II)-sulfate
[26] Bertau, M.; Müller, A.; Fröhlich, P.; Katzberg, M.: Industrial Inorganic Chemistry, Wiley-VCH, 4th ed. (2013)
[27] https://de.wikipedia.org/wiki/Chloridverfahren
[28] https://de.wikipedia.org/wiki/Titan(IV) chloride
[29] https://utopia.de/ratgeber/titandioxid-warum-du-denstoff-meiden-solltest/
[30] https://de.wikipedia.org/wiki/Titanate
[31] https://www.chemwhat.de/Tetrabutyltitanat-cas-5593-70-4/
[32] https://spectrabase.com/compound/D83YMly4Eea
[33] https://de.wikipedia.org/wiki/Vorlage:Navigationsleiste_Titanhalogenide
[34] https://de.wikipedia.org/wiki/Cermet_(materials engineering)
[35] https://de.wikipedia.org/wiki/Titan(IV)-sulfide
[36] https://en.wikipedia.org/wiki/Titanium_diselenide
[37] https://www.2dsemiconductors.com/titaniumditelluride-tite2/
[38] https://de.wikipedia.org/wiki/Titan:Saphir


