The experiments using saturated steam as a medium for cleaning surfaces, including the exact steam boiler design, have been presented in the last three episodes of the series. The final details before the presentation of the test results follow in this part.
Drying the cleaned surface
The temperature difference between the object surfaces and the room dries the object surface by convection as a result of the hot steam cleaning of the object surface from foreign particles. The free foreign particles extracted in the swath are dry and the risk of sticking is minimal.
Saturated steam and superheated steam in a vacuum
Superheated steam in a vacuum acts as a cleaning agent on the object surface and hardly causes a drop in vacuum pressure in the closed chamber during cleaning. The condensing superheated steam itself generates negative pressure as it cools down. The condensing water and the...Continued on p. 314
...foreign particles dissolved in the water follow the gravitational force of the earth, i.e. they collect at the lowest point of the vacuum chamber. If the vacuum chamber is emptied of condensate with dissolved foreign particles by a series connection of at least two drain systems, the vacuum in the chamber is hardly changed. The vapor expands from a temperature of 170 °C to 180 °C and 6.5 bar to 7 bar into the vacuum chamber. The vacuum chamber has a vacuum of at least 140 mbar to 990 mbar. These conditions mean that the expanding steam hardly cools down measurably and the cooling of the steam creates negative pressure by reducing the volume of steam to condensate water. The jacket of the vacuum chamber is insulated against heat loss.
Dissolving oil and grease films, drawing soaps and waxes
Layers and films such as oils become more fluid on the object surface at temperatures above 85 °C. Due to the hot steam pressure higher than 2 bar overpressure on the object surface, the film thickness on the surface is reduced by running off and partially blown into the adjacent room. The room has a negative pressure of at least 140 mbar. The hot steam follows the suction direction into the collection tank as a swath with the cleaned foreign particles. Contamination of the area surrounding the cleaning position on the surface by released foreign particles is prevented. Chip filters largely separate the foreign particles.
The collection of dissolved oil and grease particles as well as other loose particles
Foreign particles loosened from the object surface must be actively captured and then safely and actively separated. If this is not done, they will migrate to surrounding surfaces and settle there. There is a particular danger for humans. Inhalation and free skin surfaces separate the migrating foreign particles. Work areas in mechanical and plant engineering must therefore be equipped with various retention and separation devices for all types of foreign particles.
In contrast to open lance-Kärcher systems, where the foreign particles drift into the environment, dry steam cleaning works as a closed system. Vapors and dirt are collected.
The charge behavior of water and steam - electrical conductivity
Electrical conductivity is a measure of the quantity of dissolved charges in water. The ion content, positive cations and negative anions, are quantified. If there are more ions in the water, the current is conducted away with less resistance, an electrical conductivity according to DIN EN 27888. The term electrical conductance is less suitable in everyday usage, it is the reciprocal of the ohmic resistance and is given in Siemens.
The electrical conductivity is the reciprocal of the specific electrical resistance and is given in S/m (Siemens per meter).
Distilled water has a conductivity of around 0.055µS/cm, which is caused by the autoprotolysis of the water. Zero is not possible. The hardness builders calcium and magnesium as cations and the anions such as sulphates, hydrogen carbonates or chlorides reduce the electrical resistance in the water. Untreated water has these components to a greater or lesser extent, so natural water is conductive.
If the anode and cathode are mounted in the water, an electric current flows until the ion migration is complete. The temperature of the water influences the level of resistance of the conductor, increasing temperature reduces the resistance. Therefore, the statements about the temperatures in the measurements must be related to the bath temperature or the vapor temperature.
The quantitative measurement in µS/cm is not differentiated according to the type of dissolved ions, only the quantity of ions is counted.
The conductance G is measured in µS and is the reciprocal value of the electrical resistance R. If this conductance is multiplied by the cell constant of the measuring device k=distance/area, the result is the electrical conductivity L =k*G in µS/cm. Many measuring devices have the value for k=1, so we usually only talk about the conductance.
If the conductance is multiplied by the value 0.65, the result is the concentration of dissolved solids (TDS=Total Dissolved Solids).
If a liquid conducts an electric current, then ions must be present in this liquid through whose migration the current transport can take place. Conductivity measurements on the purest water result in a specific conductivity of æ(H2O) = 4-10-8 (Ohm)-1 cm-1 (about 1/100 of the value of ordinary distilled water). Continuation on p. 317
Ions must therefore be present in low concentrations even in the purest water. The ions are formed by proton transfer from one water molecule to another:

This process is called autoprotolysis of water (according to Brönsted). The autoprotolysis equilibrium is completely on the left-hand side of the equation. Further protolysis
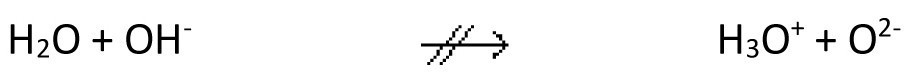
does not play a role, i.e. free oxide ions are not stable in aqueous solution.
For [H3O+] > [OH-] the solution reacts acidically, pH < 7
For [H3O+] = [OH-] the solution reacts neutrally, pH = 7
For [H3O+] < [OH-] the solution reacts alkaline, pH > 7
The electrical conductivity of the water and steam can be varied using an ion exchanger or a reverse osmosis system or by adding hardening agents. Applying an electrical voltage changes the position of the autoprotolysis equilibrium.
The equilibrium constant KW is called the ion product of the water. Its value depends on the temperature; at 25°C it is 1-10-14 mol2 l-2.
KW= 55*10-14 mol2*L-2 at 100 °C
Increasing the temperature of the water (Table 1) causes the equilibrium constant to increase. The electrical charge of the water/steam in the form of the H3O+- ions changes. This allows acidic, neutral and alkaline aqueous solutions to be characterized as shown in Table 2.
|
T/°C |
KW/mol2l-2 |
|
10 |
0.681 - 10-14 |
|
20 |
0.929 - 10-14 |
|
25 |
1,008 - 10-14 |
|
30 |
1,469 - 10-14 |
|
40 |
2,919 - 10-14 |
|
50 |
5,474 - 10-14 |
|
60 |
9,610 - 10-14 |
|
100 |
55 * 10-14 |
|
Acidic |
neutral |
alkaline |
|
|
c(H3O+) in mol/L |
> 10-7 |
10-7 |
< 10-7 |
|
c(OH-) in mol/L |
< 10-7 |
10-7 |
> 10-7 |
|
pH |
< 7 |
7 |
> 7 |
|
pOH= |
> 7 |
7 |
< 7 |
Water at higher temperatures reacts acidic. This property is effective during HP purification. According to the principle of Le Chatelier and Braun, the energy balance is calculated as to whether the autoprotolysis of the water is exothermic or endothermic.
H2O(l) + H2O(l) ↽--⇀ H3O+(aq )+ OH-(aq)
ΔrHm= [ΔfHm(H3O+) + ΔfHm(OH-)] -
[2-ΔfHm(H2O)]=[(-286.03) + (-230.09)] kJ/mol -
[2-(-286 kJ/mol] = +55.94 kJ
The reaction is therefore endothermic. According to the principle of Le Chatelier and Braun, an increase in temperature promotes the endothermic reaction, i.e. the formation of more oxonium and hydroxide ions. This is also associated with an increase in Kc, which results from the law of mass action. The Kc is only constant for a given temperature. The increase in Kc is also accompanied by an increase in KW, as the following applies:
KW=K-c2(H2O)
With the described increase in the oxonium ion concentration, the pH value decreases, but the water does not react acidically, as the oxonium ion concentration and the hydroxide ion concentration are still the same. It becomes measurably positively charged [2].
Minerals in water and steam that characterize water hardness
The water hardness indicates the ion concentrations of calcium and magnesium dissolved in the water. The higher the proportion of calcium and magnesium in the water, the higher the water hardness, the "total hardness". A German degree of hardness (1 °dH) corresponds to 10 mg of calcium oxide or 7.19 mg of magnesium oxide per liter of water.
Hot water and heating systems require soft water to counteract the formation of boiler scale. The dosage of detergents also depends on the water hardness, as various surfactants, in particular linear alkylbenzene sulphonates (LAS), have a hardness-dependent effect. The hardness formers reduce the washing power of detergents. Appropriate dosage instructions must be indicated on the detergent packaging. Soap foams poorly in hard water because it forms insoluble calcium and magnesium salts. The effect of water hardness is therefore mitigated by most detergents by adding phosphate. In technology, softening is carried out by means of:
- Distillation
- Precipitation with soda or sodium phosphate
- with ion exchangers using so-called softening systems
- in the household through the softening agents built into detergents and cleaning agents.
On February 1, 2007, the German Bundestag passed the new version of the law on the environmental compatibility of detergents and cleaning agents (Detergents and Cleaning Agents Act - WRMG). The new version came into force on May 5, 2007 (see Federal Law Gazette Part 1 of May 4, 2007, p. 600).
According to Section 9 of the Act, water supply companies are obliged to indicate the hardness ranges of drinking water to consumers as follows:
- Hardness range soft: less than 1.5 mmol calcium carbonate per liter (corresponds to 8.4 °dH)
- Hardness range medium: 1.5 to 2.5 mmol calcium carbonate per liter (corresponds to 8.4 °dH to 14 °dH)
- Hardness range hard: more than 2.5 mmol calcium carbonate per liter (corresponds to more than 14 °dH)
These new three hardness ranges replace the old four ranges. The information must be given in millimoles (mmol) of calcium carbonate per liter (which is internationally standard for hardness information). It is assumed that the total hardness (sum of the concentrations of calcium and magnesium, calculated as calcium carbonate) must continue to be stated. However, the law makes no statement on this.
The new hardness ranges are based on European law; the EC Detergents Regulation obliges detergent manufacturers to provide dosage recommendations for these three hardness ranges. As different definitions of water hardness were used in France, England and Germany, this term was redefined in the course of EC-wide standardization and now only refers to the content of calcium and magnesium ions.
Water hardness is the concentration of Ca2+ and Mg2+ ions c(Ca2++ Mg2+) in mmol per liter (DIN 38409-H 6).
In general, 70-85 % of the total hardness consists of calcium hardness and 30-15 % of magnesium hardness. A very common and frequently used subdivision of water hardness, which continues to play an important role in both water management and construction, is based on the anions present. A distinction is made between carbonate and non-carbonate hardness.
Carbonate hardness (temporary hardness): The carbonate hardness (KH) is that proportion of calcium and magnesium ions for which there is an equivalent concentration of hydrogen carbonation in the unit volume. The KH can be removed by boiling.
Ca2++ Mg2++ 4 HCO₃ - → CaCO₃ ↓ + MgCO₃ ↓ + 2 H₂O + 2 CO₂<1>
Non-carbonate hardness (permanent hardness or residual hardness): The non-carbonate hardness (NKH ) is the residual Ca2+ and Mg2+ ions that may remain after deducting the KH from the total hardness, which mainly originates from the dissolution of sulphates (e.g. from CaSO₄) and chlorides (e.g. CaCl₂). The NKH cannot be removed by boiling. The determination of water hardness is one of the most common routine determinations in both the analytical and technical fields. In Germany, water hardness is often still specified in degrees of German hardness °dH or °d.
The following applies: 1 °dH = 10 mg CaO (or 7.14 mg MgO) in 1 liter of water.
The problem with this definition is that the concentration of hardness constituents is attributed to the CaO content, although this compound is not a water constituent at all.
Therefore, if the hardness of the water is to be determined for a specific problem and specified in accordance with DIN, only the concentration of the hardness formers (calculated as calcium) in mmol per liter can be considered. As this value is often not clear enough, °dH should also be specified.
According to the required specification of the water hardness in mmol/l (1 mmol (Ca2++ Mg2+)/l = 5.6 °dH), the following hardness ranges result: Hardness range c(Ca2++ Mg2+) /l H2O°dH
1 (soft) < 1.3 mmol < 7
2 (medium hard) 1.3-2.5 mmol 7 - 14
3 (hard) 2.5-3.8 mmol 14-21
4 (very hard) > 3.8 mmol > 21
As the amount of Mg2+ is usually small compared to the Ca2+ concentration in the water and both cations are determined analytically together, the magnesium content is usually not shown separately but added to the Ca2+ content.
Part 9 contains an overview of the tests and test results as well as technological parameters for successful surface cleaning
Literature
[1] http://groups.uni-paderborn.de/cc/lehrveranstaltungen/_aac/vorles/skript/ kap_10/kap10_2.html, Prof. Dr. Gernot Reininger and Prof. Dr. Volker Schubert, University of Paderborn
[2] www.jagemann-net.de/massenwirkungsgesetz/massenwirkungsgesetz.php
M. Jakob; S. Erk: Der Wärmeübergang beim Kondensieren von Heiß- und Sattdampf, Mitteilungen aus der Physikalisch-technischen Reichsanstalt, Heft 310, H4, VDI-Verlag Berlin, 1928


