The energy transition calls for energy sources that are climate-friendly, i.e. that cause as littleCO2 emissions as possible during production and use - ideally none at all. Synthetic energy sources - i.e. those that are obtained from renewable energy through conversion processes - are one way of achieving this. This is because the use of such energy sources only generates just as muchCO2 as was previously extracted from the atmosphere for their production. Artificially produced methane falls into this category.
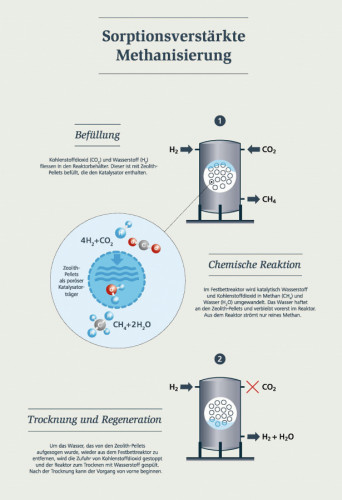 Sorption-enhanced methanation: filling, chemical reaction, drying and regeneration (Graphic: Empa)Synthetic gas offers enormous potential if it is produced from atmosphericCO2 and renewably generated hydrogen. However, in addition to renewable electricity, hydrogen production also requires a lot of water. In the "move" mobility demonstrator, water for hydrogen production is therefore to be extracted directly from the atmosphere on site in addition toCO2 with the help of aCO2 collector from the spin-off "Climeworks" at ETH Zurich. Such concepts could then also be implemented in desert regions without liquid water supplies in the future.
Sorption-enhanced methanation: filling, chemical reaction, drying and regeneration (Graphic: Empa)Synthetic gas offers enormous potential if it is produced from atmosphericCO2 and renewably generated hydrogen. However, in addition to renewable electricity, hydrogen production also requires a lot of water. In the "move" mobility demonstrator, water for hydrogen production is therefore to be extracted directly from the atmosphere on site in addition toCO2 with the help of aCO2 collector from the spin-off "Climeworks" at ETH Zurich. Such concepts could then also be implemented in desert regions without liquid water supplies in the future.
However, the production of synthetic methane from hydrogen andCO2 - known as methanation - has its pitfalls. This is because the gas produced in a catalytic process still contains hydrogen, which makes it impossible to feed it directly into the gas grid. Empa researchers have therefore developed a new reactor concept in which the formation of hydrogen on the product side is prevented. This enables Empa researchers to achieve simpler process control and better suitability for dynamic operation, e.g. for coupling with intermittently available renewable energies.
The hydrogen-free methane is produced in the "move" with so-called sorption-enhanced methanation. The idea behind this: The water produced during the reaction is continuously adsorbed on a porous catalyst carrier during the methanation process. This continuous removal of water means that the only product is methane - in its pure form. This eliminates the need to purify the (previous) product mixture. At the end of the reaction process, the catalyst carrier material is dried again by reducing the pressure - and is ready for the next reaction cycle.
The regeneration time, i.e. the time required to dry the reactor, is crucial for reactor design and process planning. To ensure continuous methane production, at least two reactors must therefore operate alternately. Suitable heat management is also essential for drying the reactors, either by dissipating the heat from the reactor or by storing heat internally in the catalyst bed.


