Question: For some years now, one of our rack systems has mainly been used for direct tinning of non-ferrous metals. Apart from the coating thickness and the adhesive strength, the only requirement so far has been that the parts should have a nice shine. Recently, the number of complaints has increased. Hair is growing from the surface; according to the literature, these are typical whiskers. One of the problems we are facing is the age of the parts that are the subject of complaints. They date back up to two years, which is why we find it difficult to re-galvanize them as a gesture of goodwill. Another problem is finding a permanent technical solution. We were advised to do without organic additives, but this has a massive impact on the appearance. Another piece of advice was to anneal the parts, which also has a negative impact on their appearance. Our position is that we are basically not liable if the parts are that old. In addition, any measure would have a negative impact on the primary requirement, namely the appearance. Would you agree with that?
Answer: No. As far as the acceptance of older, rejected parts is concerned, there are very different regulations and agreements. In most cases, whisker formation is due to electroplating or can be prevented, as we will describe in more detail below. Legally, you may be able to avoid a large proportion of the rejected items, but you run the risk of losing the customer (or several) completely. We therefore strongly advise you to work together constructively and to book part of the costs as "learning money". It has often been shown that customers contribute to the costs in such cases if they receive quick and competent help and also have the feeling that the electroplating company is playing with open cards. But now to the technical problem.
What are whiskers?
Whiskers are thread-like crystals that grow out of a metal or metal layer over time and resemble cat whiskers. Over time, they can become several millimeters or even centimeters long. Apart from being unsightly, they can also cause technical problems, e.g. short circuits in electronic components.
Formation of whiskers
Three conditions must be met for this to happen:
- Internal stresses, i.e. compressive stress
- Sufficient temperature for diffusion
- Time.
As long as a compressive stress exists and the temperature is sufficient, whisker formation can occur. Depending on the overall constellation, this may take a longer period of time, but the problem remains.
Whiskers are preferably formed by diffusion between the layers (e.g. in the case of undercopper plating) and the substrate, i.e. the non-ferrous metal. Growth can continue for several months or even years, with intermetallic phases forming. Whether and to what extent whiskers form depends on various factors. The electrolyte, the deposition conditions or parameters, the thickness and type of interlayers, the substrate and the post-treatment, to name but a few.
Intermetallic phases
Intermetallic phases (IMP) are chemical compounds of two or more metals that have a lattice structure that differs from that of the constituent metals. This lattice structure consists of a mixed bond of metallic and lower atomic or ionic bonds. There are IMPs with a stoichiometric composition according to the usual valences of the metals and there are also IMPs that have more or less extended homogeneity ranges in the phase diagram. These homogeneity ranges, also known as phase widths, indicate the range in which the quantity ratio of the different metals can vary. Compared to ordered phases, IMPs are more stable, but hard, brittle and poor conductors of electrons. With increasingly simpler ratios and narrower homogeneity ranges as well as simpler lattice types, the resonance to a non-metallic bonding component increases. Due to the modified crystal structure, IMPs have different physical and chemical properties such as hardness or contact resistance.
A few examples:
Cu6Sn5: hexagonal η-phase
Cu3Sn: hexagonal closest packed ε-phase
Ni3Sn4: stable phase, monoclinic structure
NiSn3: metastable phase, which is not described in any Ni-Sn phase diagram.
Diffusion describes the process by which individual atoms, ions or particles migrate through the lattice to follow paths that are greater than the atomic distance. This leads to macroscopic mass transport. In metals, this penetration in the solid state is of particular importance. It is a statistical process in which the migration of atoms takes place due to differences in concentration. There are different types of diffusion:
- Volume diffusion
- grain boundary diffusion
- surface diffusion.
To enable diffusion in a solid, it is necessary to temporarily increase the energy of the diffusing atom. This process requires an activation energy. Volume diffusion requires a higher activation energy than grain boundary diffusion or surface diffusion.
In substitutional solid solutions, in which the lattice sites are predominantly occupied, the vacancy mechanism is the predominant mechanism for diffusion. Here, an atom jumps to a neighboring vacancy and leaves a vacancy for another atom. The diffusion coefficient, which determines the growth, depends on various factors such as the type of material, the concentration, the temperature and the diffusion path.
The electrolyte
In order to counteract the formation of whiskers, electrolytes containing lead were used in the past, as the formation of whiskers can be massively counteracted from a lead content of just 1% in the layer. Unfortunately, this is only possible in exceptional cases nowadays.
Newer tin electrolytes are already being developed in such a way that whisker formation is minimized if the specified parameters are adhered to. These electrolytes generally contain fewer organic additives and form layers with larger crystals. It is important that the layers are as free as possible from residual stresses and that there is either no or very uniform diffusion.
For this reason, matt tin electrolytes are used almost exclusively in the electronics sector. The gloss is later produced by the reflow process. In this process, the tin layer is heated above the melting point for a short time, which works particularly well with strip electroplating. As soon as the tin layer solidifies, the previously very soft and matt tin layer is somewhat harder and shiny. Remelting is carried out to remove the compressive stress from the layer and immediately form an IMP, which then only grows very slowly, if at all. This will probably not be an option for your rack parts, but at least explains the suggestion you listed to anneal the parts. This is also done in practice, e.g. hot-dip tinning of strips, to allow the IMP to grow. Annealing does not cause the coating to melt, but it should be carried out in an oxygen-free oven so as not to negatively affect the appearance. You should ensure that you have an up-to-date electrolyte type. In addition, the bath parameters, Sn(IV) content and organic content should be checked and corrected.
Intermediate layers
There are various reasons for using interlayers in the electronics sector, which we will not go into in detail here. In order to effectively prevent or at least minimize whiskers, we strongly recommend nickel plating before tinning. Effective protection already occurs from a layer thickness of 0.5 µm. Over time, NiSn3 and Ni3Sn4 IMPs form and the nickel layer is a barrier layer, also known as a diffusion barrier. The IMP that forms is therefore very small, the growth rate is around a quarter of a Cu6Sn5 phase, but such an intermediate layer should not be seen as an all-purpose weapon. If there are additional problems with the tin electrolyte, whiskers can form at a higher tin layer thickness even despite the nickel interlayer. It is important to pay attention to all of the above points and to get the supplier of the bath chemistry on board.
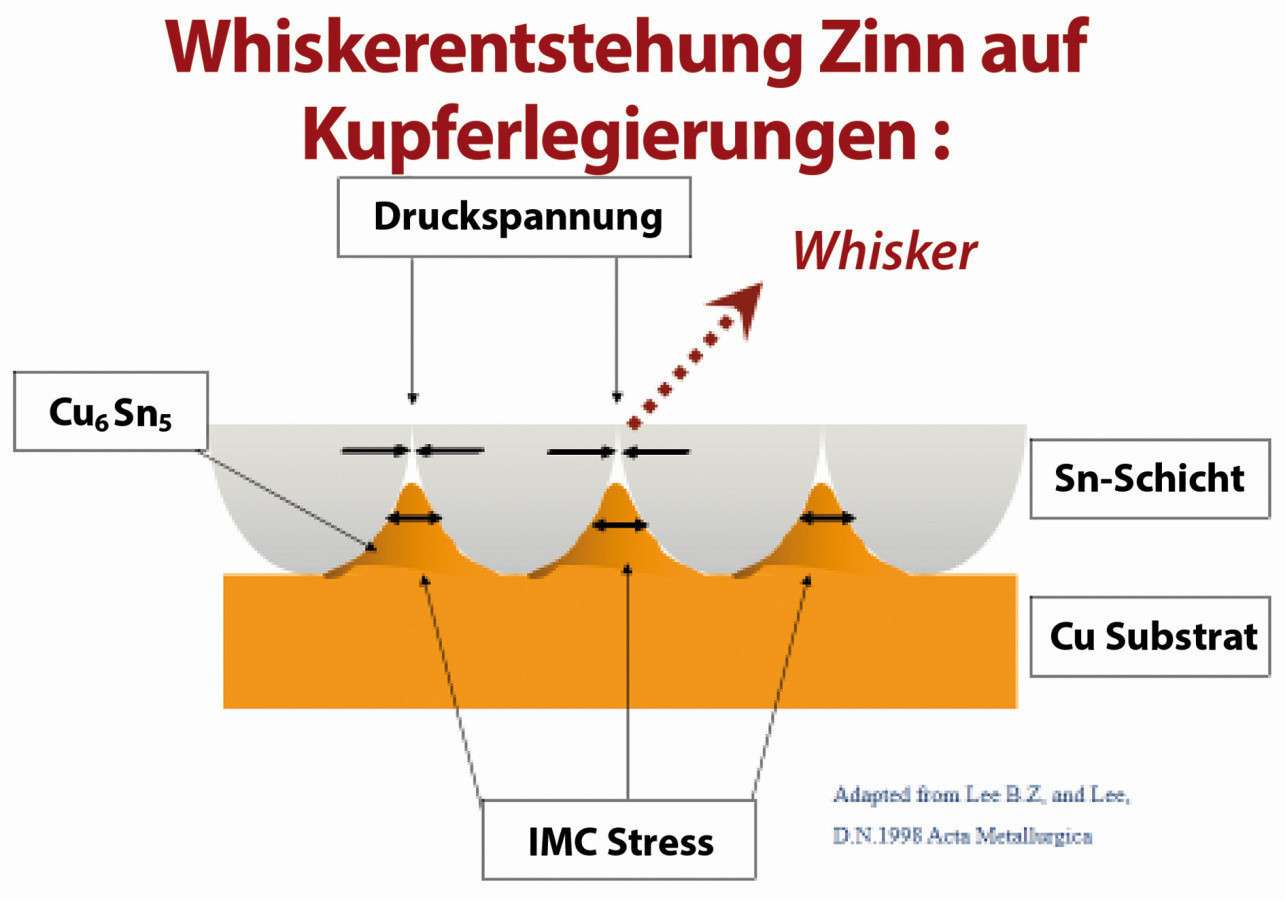 Whisker growth due to the intermetallic layer
Whisker growth due to the intermetallic layer
Processing the complaint
In addition to the measures regarding the electrolyte composition and its condition, you must clarify whether a nickel interlayer is permissible. In terms of costs, we believe that you even have good leverage in negotiations with the customer if there is no mention of pre-nickel plating on the drawings and orders, as this is essential to prevent whisker formation during galvanic tinning. Point out the technical necessity to the customer, even if the costs for the coating will increase. Ask him to modify the drawings accordingly for the future, and we also consider it good practice to inform the customer of the measures taken with regard to the electrolyte. In our experience, this restores any lost trust.


