Is the dream of hydrogen over?
The dream of hydrogen as the engine of a new, sustainable economy, e.g. in the production of so-called "green" steel? The enthusiasm that has been so great in recent years seems to be fading. Take ArcelorMittal, for example, one of the world's largest steel manufacturers. Five years ago, the company issued a press release stating that it wanted to invest in new production technologies in order to become climate-neutral by 2050. The direct reduction of iron ore with natural gas, which has been tested at the Hamburg site, is therefore to be realized with hydrogen in the future - initially via steam reforming of natural gas until sufficient green hydrogen is available to produce steel in a climate-neutral way.
The plan is to produce 100,000 tons of steel using hydrogen as early as 2025. A win for the environment, because hydrogen in the DRI (Direct Reduced Iron) process can be used to produce steel almostCO2-free- hydrogen is a real game changer for the decarbonization of the steel industry [1].
On June 19 of this year, the Luxembourg steel giant admitted the failure of this plan: "It is becoming increasingly clear that the energy transition is progressing more slowly than expected in all areas. This includes the fact that green hydrogen is not yet a viable energy source and that natural gas-based DRI production is not competitive as a transitional solution." [2]
The withdrawal means that ArcelorMittal Germany is foregoing billions of euros in public funding for the implementation of the new technology - "but even with the financial support, the economic viability of this conversion is not sufficient," summarizes Geert Van Poelvoorde, CEO of ArcelorMittal Europe.
Green hydrogen
The idea of anchoring green hydrogen as a cornerstone of the energy transition arose from two key findings:
- Many applications cannot be directly electrified - be it in heavy industry, shipping, aviation or blast furnaces. Green hydrogen, produced by electrolysis with electricity from wind and sun, can replace fossil fuels in these sectors and thus enable significantCO2 reductions. Due to this potential, it has been considered a key technology for a climate-neutral energy system since the mid-2010s, which is reflected in the EU hydrogen strategy and Germany's national hydrogen strategy.
- The expansion of renewable energies has brought with it the problem of generation peaks and dark doldrums. Green hydrogen makes it possible to convert surplus electricity from wind and solar parks into chemical energy, store it and call it up again with a time delay (power-to-gas). This flexible storage and transportation function has made it an indispensable component of a supply based on 100% renewable energies.
However, the technological transition to a hydrogen economy based on green hydrogen is not progressing as quickly as politicians would like in view of ambitious climate targets and the desire for a resilient energy supply.
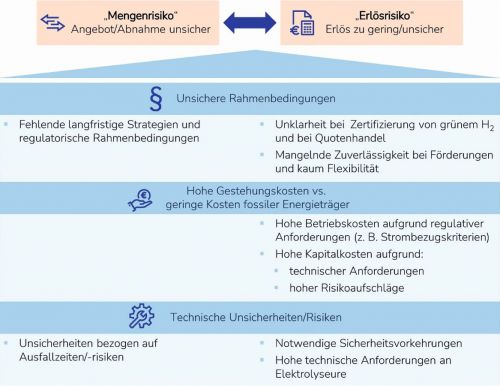 Fig. 1: Overview of challenges and interactions in the ramp-up of a hydrogen economy in Germany (graphic: VDI)
Fig. 1: Overview of challenges and interactions in the ramp-up of a hydrogen economy in Germany (graphic: VDI)
In April of this year, the Association of German Engineers (VDI) called for more "pragmatism instead of overregulation" in the ramp-up of hydrogen. Despite clearly formulated goals for the promotion of green hydrogen by the German government, too little has happened in practice so far. "Although the coalition agreement contains some positive signals - including faster approval procedures - many things are still too vague. For example, the further promotion of hydrogen use and production by reducing the tax burden," criticizes VDI Director Adrian Willig. And Michael Sterner, professor at OTH Regensburg and VDI hydrogen expert, recognizes a "chicken and egg problem" during the ramp-up: "Both potential producers and users of green hydrogen and its derivatives are confronted with substantial purchase and supply risks as well as high revenue risks." VDI Director Adrian Willig confirms: "We now need coordinated political support that specifically addresses both risks - beyond 2030."(Fig. 1)
Fast career of a light element
Hydrogen has been known since 1766. In his publication "Experiments on Factitious Air", Henry Cavendish reports how he allowed hydrochloric acid to act on iron, zinc and tin in an experiment. The result was hydrogen, which he described as "inflammable air", or phlogiston.
Hydrogen has been known since 1766 and the lightest and first element in the periodic table of elements has been inspiring minds ever since.
Since then, the lightest and first element in the periodic table of elements has been inspiring minds. On December 1, 1783, the French physicist Jacques Charles and his co-pilot Nicolas-Louis Robert made the first manned flight with a hydrogen balloon in Paris, shortly after they had launched the first unmanned hydrogen balloon in August. And just two years later, on January 7, 1785, Jean-Pierre Blanchard and John Jeffries crossed the English Channel by air in a hydrogen balloon.
The invention of the hydrogen economy
 Fig. 2: In his novel "The Mysterious Island", Jules Verne postulates a future hydrogen economy as early as 1874 (Photo: Amazon)
Fig. 2: In his novel "The Mysterious Island", Jules Verne postulates a future hydrogen economy as early as 1874 (Photo: Amazon)
Could a replacement be found? "But what could be found?" asks the character Pencroff, "do you have an opinion on that, Mr. Cyrus?" - "A superficial one, yes, my friend. - Well, what will serve in place of coal for burning? - Water, replied Cyrus Smith. - The water! exclaimed Pencroff in astonishment; water to propel steamboats and locomotives, water to heat water with? - Yes, but water broken down into its elementary constituents, Cyrus Smith instructed him, broken down by electricity, which by this time will have grown into a powerful and easily usable force, for all great inventions seem to add to themselves at the same time in consequence of an inexplicable law. I am convinced, my friends, that water will one day be used as a fuel, that hydrogen and oxygen, its constituents, will become an inexhaustible source of heat and light of an intensity quite undreamed of. The day will not fail to come when the coal chambers of steamers and the tenders of locomotives will carry these two gases instead of coal, perhaps in a compressed state, which will develop an enormous heating power under the boilers. So have no fear!" (Fig. 2)
This short dialog in the novel "The Mysterious Island" is astonishingly topical. On the one hand, it testifies to the optimism of the engineers and their confidence that it will always be possible to find something new and make it usable when a certain resource runs out.
On the other hand, the passage makes it clear that forecasts about future technical developments are fundamentally based on the state of the art at the time of the forecast and that only the technical possibilities available at the time are extrapolated into the future. Forecasts cannot take into account "disruptive factors" such as new discoveries and associated innovations that give the development of technologies a completely different spin. You can't know what you can't know. Jules Verne, for example, could not have known in 1874 that Carl Benz would invent the first practical car with an internal combustion engine in 1886, which a few decades later would overtake the railroad on the basis of inexpensive crude oil. The prospect of a shortage of coal depressed him.
 Fig. 3: The illustration visualizes a possible production platform for offshore hydrogen. Background information: https://idw-online.de/de/ news853540 (Photo: Helmholtz-Zentrum Hereon / Aquaventus Förderverein e.V.)
Fig. 3: The illustration visualizes a possible production platform for offshore hydrogen. Background information: https://idw-online.de/de/ news853540 (Photo: Helmholtz-Zentrum Hereon / Aquaventus Förderverein e.V.)
All the more astonishing is the clarity with which Verne anticipated the economic significance of hydrogen - produced electrolytically from water. He was familiar with electrolysis; the first documented electrolysis of water was carried out in 1800 by the British scientists William Nicholson and Anthony Carlisle. They used Volta's column, which had been invented shortly before, to conduct an electric current through water. They observed the release of two gases in a 2:1 ratio, which they correctly identified as hydrogen and oxygen (Fig. 3).
Hydrogen as the key to decarbonization
It is actually incomprehensible that hydrogen, which is now on everyone's lips as a "new discovery" and is seen as indispensable for the climate-friendly conversion of energy-intensive sectors such as steel, chemicals, cement and gas-fired power plants, had a shadowy existence after the Second World War. It must have been the aftermath of the Lakehurst disaster and the associated trauma that led people to abandon hydrogen: The Zeppelin LZ 129 "Hindenburg" was destroyed on May 6, 1937 when it landed in Lakehurst (New Jersey, USA) when the hydrogen filling ignited. 35 of the 97 people on board and one member of the ground crew lost their lives.
On the other hand, from the 1950s onwards, the alternative energy source was cheap crude oil, which seemed to flow endlessly until the so-called oil shock at the turn of 1973/74. And it was a mainstream belief in technology, business and politics that nuclear energy would increasingly replace fossil fuels such as coal, oil and gas from the 2000s onwards.
There were only a few who thought outside the box. One of them was Reinhard Dahlberg, whom I met in Heilbronn at the beginning of 1980. At the time, Dahlberg was head of the semiconductor division of AEG-Telefunken, which was based there. Today, his name has almost been forgotten, but he deserves the honor of being one of the first to consistently formulate the idea of producing hydrogen with photovoltaics and using it as an energy source. In an interview in 1990, he recalled: "We have been building solar cells for space travel since 1963. I myself brought some well-known German specialists in this field back from the USA. And then one day the German government approached us and said that we should set up a research program on terrestrial solar cells together with Wacker-Chemie and AEG's sister company in Wedel. At the time, I thought what nonsense that was. But I got to grips with the task. And the more I got into it, the more I became convinced that the way the concept was conceived (namely that solar cells would be used to generate electricity in Germany, perhaps even in competition with nuclear energy) was economic nonsense. And when I was ready, I asked myself the question: "What would be the right way to do it?"
In 1980, under the impression of the oil crisis, the physicist developed the concept of a hydrogen economy in which hydrogen is produced with solar energy in desert areas in order to bring it to consumers via pipelines as a transportable primary energy source. I published six installments of Dahlberg's concept when I was editor of the newspaper VDI nachrichten [3].
The Dahlberg concept was fascinating, but was never considered even remotely feasible in business and politics. Dahlberg had not only described a vision that could have been agreed to without obligation, but also meticulously calculated everything. His essentials were as follows:
- Technical: use of solar energy solely through photovoltaics
- Spatially: use solely in large centralized plants with solar farms
- In terms of time: building a global system of hydrogen economy through self-reproducing automatic factories
He estimated that the transformation to a global hydrogen economy would take many decades into the middle of the 21st century. And Dahlberg estimated the total investment costs for the project to cover the world's energy needs at around 90 trillion German marks.
That was probably the concept's hitch. People did not want to think about the scale of the approach and its political implications - Dahlberg had envisaged the Saharan states as a location for photovoltaics. "The concept is in our bottom drawer," I was told when I asked the Federal Ministry of Research and Technology (BMFT) at the time. In the 1980s, Dahlberg's figures were weak arguments against the further development of nuclear energy use as the central anchor of future energy independence and decarbonization.
Hydrogen à la carte
The hydrogen economy project is technically well founded. The H2 molecule is non-toxic and non-flammable. It is 14 times lighter than air and has a high energy density. The calorific value of 1 kg of H2 is equivalent to that of 2.8 kg of gasoline or 2.1 kg of natural gas. In short: as an energy source, hydrogen is no more dangerous than natural gas or crude oil. Dangers such as explosions and hydrogen embrittlement are technically controllable.
However, it is often underestimated how lengthy and complicated a desired system change in technology can be. Normally, innovations in technology take place in a quasi-evolutionary way according to the respective market conditions. Of course, there are also occasional revolutionary developments - leap innovations that break through due to their (economic) advantages and turn the world upside down in a short space of time. At present, the application of artificial intelligence methods probably has such disruptive potential. As a rule, however, technological changes take place gradually and almost imperceptibly, behind the backs of the players, so to speak.
The situation is completely different when a technological change is politically desired and planned. This also applies to Dahlberg's approach, who estimates that it will take around 80 years to implement his concept. As a deliberate political act, however, a change in technology is usually treacherous, because the nature of a modern technological infrastructure is to be complex and to be based on many different interactions between the technologies involved and feedback loops in society. Deliberate political intervention in the technical system can quickly have consequences that no one had thought of.
In the current debate about a future hydrogen economy, it is easy to get the impression that some players in politics and society are completely underestimating the complexity of a change in the technological infrastructure of our energy supply and the time required for this. The assumption that the energy transition is a matter of a few years and can be mastered in the blink of an eye - this bet on the future could backfire.
The German Society for Electroplating and Surface Technology (DGO) conducted a survey [4] to determine what effects a future hydrogen economy can be expected to have on the electroplating industry, according to which companies are clearly aware of the increasing importance of electroplating processes for the hydrogen economy. However, according to the DGO, there are still a few stumbling blocks to overcome. In addition to the high production costs of green hydrogen, a lack of nationwide distribution infrastructure, regulatory uncertainties and poor prospects for rapid scaling of the market, there are also technological challenges to overcome when it comes to storing and transporting hydrogen.
Technological challenges
In fact, the hydrogen atom is so small that it can literally pass through any wall. This is often associated with a degradation of the mechanical properties of the material containing the hydrogen. Hydrogen embrittlement, which is feared in materials engineering, is mainly manifested in reduced ductility. At very high concentrations, the strength of the materials can also be impaired - up to and including hydrogen-enhanced cracking.
 Fig. 4: Feeding hydrogen into the existing European gas grid is associated with technical and regulatory challenges. The Federal Institute for Materials Research and Testing (BAM) is therefore working on a comprehensive knowledge database that provides important information on standards for safe materials and components as well as on the European gas infrastructure. Background information: https://idw-online.de/de/news838264 (Photo: stock.adobe.com)
Fig. 4: Feeding hydrogen into the existing European gas grid is associated with technical and regulatory challenges. The Federal Institute for Materials Research and Testing (BAM) is therefore working on a comprehensive knowledge database that provides important information on standards for safe materials and components as well as on the European gas infrastructure. Background information: https://idw-online.de/de/news838264 (Photo: stock.adobe.com)
There is still a lot of need for research in the field of hydrogen embrittlement in particular - which is also being addressed: For example, the Federal Institute for Materials Testing (BAM) has pooled its expertise in the field of hydrogen technologies in a competence center. And with regard to the expansion plans to transport hydrogen in pipelines previously used for natural gas in the future, the German Technical and Scientific Association for Gas and Water (DVGW) has randomly examined and evaluated the hydrogen suitability of steel materials for gas pipelines and systems in a research project, analyzed the behaviour of hydrogen in the transport network and identified a transformation path to higher hydrogen concentrations in the European gas network (Fig. 4).
Research successes
In addition, the aim of basic research is to increase the level of knowledge about the behavior of hydrogen. One key question is how hydrogen embrittlement of metals can be prevented, especially in high-strength steels, which are particularly susceptible. It is known that a catalytic splitting of the hydrogen molecules into atomic hydrogen first occurs on the corresponding metal surface. The individual hydrogen atoms then penetrate the metal lattice and form alloy-like or interstitial hydrides with the metal, usually with a non-stoichiometric composition.
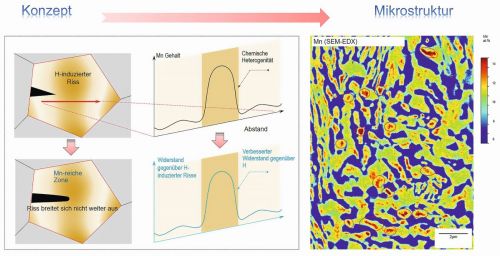 Fig. 5: Chemical heterogeneity within the microstructure results in improved resistance to hydrogen-induced cracking and suppresses hydrogen-induced premature material failure (Photo: MPI-SusMat / Nature Materials; DOI: 10.1038/s41563-021-01050-y)
Fig. 5: Chemical heterogeneity within the microstructure results in improved resistance to hydrogen-induced cracking and suppresses hydrogen-induced premature material failure (Photo: MPI-SusMat / Nature Materials; DOI: 10.1038/s41563-021-01050-y)
An international team of scientists led by Binhan Sun from the Max Planck Institute for Sustainable Materials (MPI-SusMat) in Düsseldorf has now found a way to stop hydrogen-induced cracking in high-strength steels, as the researchers report in the journal "Nature Materials". "Our goal was to find a cost-effective, scalable strategy to make high-strength steels more resistant to hydrogen while maintaining their mechanical performance," comments Binhan Sun, topic leader for hydrogen embrittlement in high-performance alloys at the MPI-SusMat and first author of the publication, on the research success, which cannot be overestimated (Fig. 5). The scientists implemented manganese-rich areas in the microstructure of the steel to blunt cracks and trap hydrogen in them, thus stopping crack propagation. "We tested our method with high-strength manganese steels in which we created an extremely high number density (over ~2 × 1018 m-3) of manganese-rich buffer zones. These buffer zones provide dead ends for cracks by blunting sharp cracks. This makes the steel twice as resistant to hydrogen as conventional chemically homogeneous steels, regardless of when and how hydrogen has penetrated the material," says Dirk Ponge, head of the MPI-SusMat group "Mechanism-based Alloy Design". The method presented in Nature Materials can, in principle, be applied to many established types of steel.
Aluminum also tends to become brittle on contact with hydrogen. Hydrogen-resistant alloys were previously too soft to be used for high-tech applications. An international research team - including scientists from the MPI-SusMat - has now found an innovative solution: They developed a design strategy that enables them to develop particularly strong alloys that are also resistant to embrittlement. The researchers report in the journal Nature on how they make aluminum fit for the hydrogen industry: using a two-stage heat treatment, fine nanoparticles of Al3Scwere first produced, enclosed in a shell of Al3(Mg,Sc)2. Both types of particles distributed throughout the aluminum-magnesium alloy fulfill two important functions: The fine Al3Scparticles increase strength, while the Al3(Mg,Sc)2 particles increase hydrogen resistance.
This new Al alloy exhibits 40 percent higher strength and five times better hydrogen embrittlement resistance compared to scandium-free alloys - even with a hydrogen load of up to 7 ppmw (parts per million by weight). Despite being loaded with hydrogen, the alloy remains ductile and does not form any hydrogen-induced cracks, the researchers conclude.
SOURCES:
[1] https://t1p.de/gfqzs
[2] https://t1p.de/xft3u
[3] Reinhard Dahlberg, Löst Wasserstoff die fossilen Brennstoffe ab?, VDI nachrichten 1980, 6 episodes, No. 31 - 36.
[4] Galvanotechnik 116 (2025) No. 3, p. 330 ff.


