Chromium and lead removal from electroplating wastewater
Scientists in the state of Madhya Pradesh in India have developed ceramic membranes, with and without chitosan coating, for the removal of chromium (VI) and lead from aqueous liquids. The ceramic membranes were molded from locally available clay. The degree of removal of heavy metals was significantly higher with the chitosan-coated membrane. While up to 65 % Cr (VI) and 68 % Pb could be removed with the normal ceramic membrane, the chitosan-coated membrane was able to remove up to 81 % Cr (VI) and 93 % Pb. The tests were carried out at initial concentrations of 55.3 mg.dm-3 Cr (VI) and 3.5 mg.dm-3 Pb and pH 3.5. The filtration pressure was 300 kPa.
The coating of the ceramic membrane with chitosan was achieved by spin coating. The paper describes the methodology and the properties of the membranes in detail. Unfortunately, the results on heavy metal removal with wastewater from electroplating factories are not described in detail.
Indian J. Chem. Technol. 2020, 27, pp. 294-302
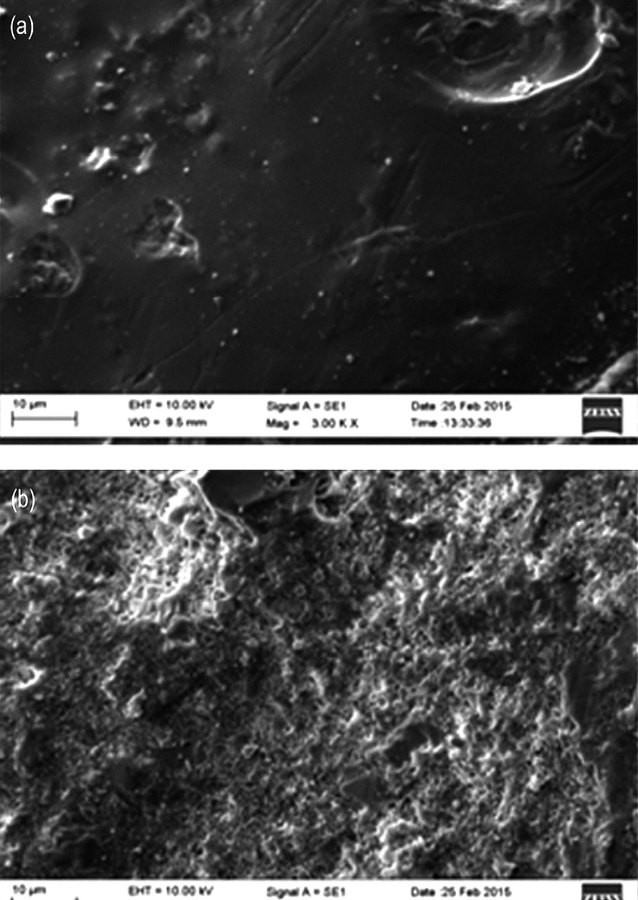 SEM images of ceramic membrane (a) with and (b) without chitosan coating
SEM images of ceramic membrane (a) with and (b) without chitosan coating
Electroplating and supercapacitors
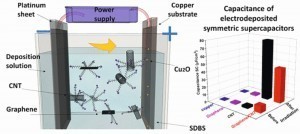 Process diagram and graphic representation of a supercapacitor. These are becoming increasingly importantWiththe exponential growth in portable devices for a wide variety of applications, the demand for power supply is increasing in parallel. Supercapacitors are ideal for systems such as medical monitoring devices or motor vehicles that require a powerful auxiliary power supply, especially for short periods of time. The supercapacitor bridges the gap between the electrolytic capacitor, in which the electrolyte is the cathode, and the battery. The conductive connection between two electrodes in a supercapacitor is the electrolyte. Supercapacitors have either symmetrical or asymmetrical electrodes.
Process diagram and graphic representation of a supercapacitor. These are becoming increasingly importantWiththe exponential growth in portable devices for a wide variety of applications, the demand for power supply is increasing in parallel. Supercapacitors are ideal for systems such as medical monitoring devices or motor vehicles that require a powerful auxiliary power supply, especially for short periods of time. The supercapacitor bridges the gap between the electrolytic capacitor, in which the electrolyte is the cathode, and the battery. The conductive connection between two electrodes in a supercapacitor is the electrolyte. Supercapacitors have either symmetrical or asymmetrical electrodes.
Scientists at the Institute of Nanoscience and Nanotechnology in Attikis, Greece have developed stable, aqueous dispersions of graphene, carbon nanotubes (CNTs) or their mixtures, with sodium dodecylbenzenesulfonate as the dispersing agent. These were used as electrolytes to electrophoretically form copper films with dispersed particles. When these Cu films were irradiated using a xenon lamp, the properties of the Cu films changed positively with respect to symmetric supercapacitors. Symmetrical solid-state supercapacitors could be fabricated using a gel electrolyte and the copper electrodes with dispersed graphene or CNT particles described above.
The best electrochemical properties (surface capacitance 78.8 μF.cm-2, energy density 0.04 μW.h.cm-2 and power density 63.03 μW.cm-2) were achieved with irradiated Cu-CNT electrodes. The electrolytic co-deposition of graphene or CNT in the copper layer is scalable.
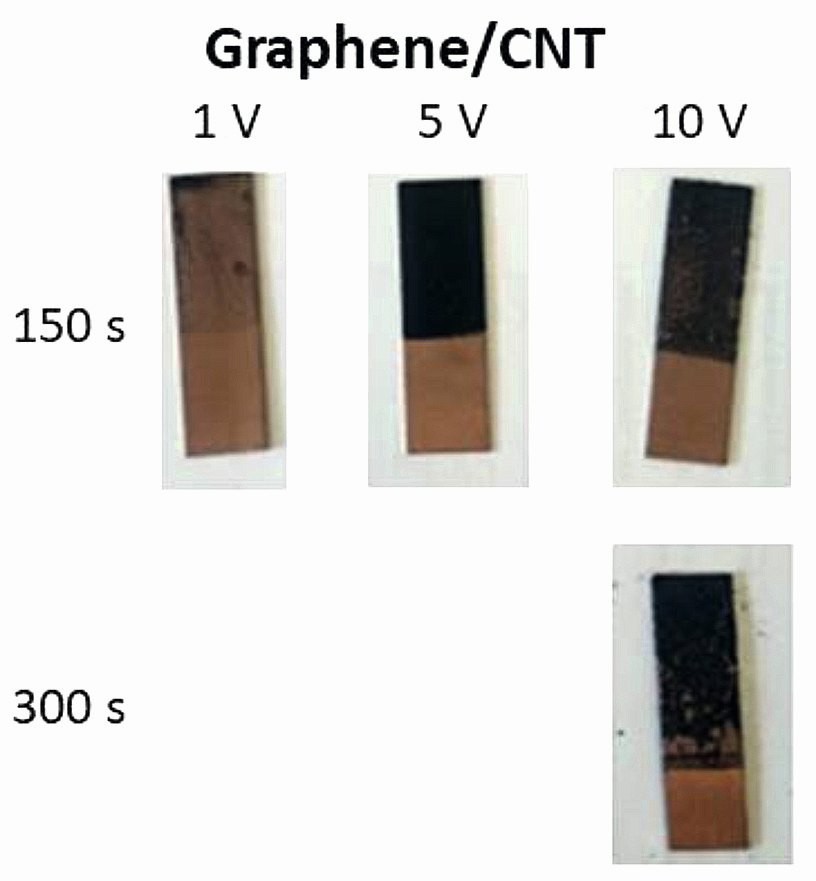 Graphene/CNT mixture electrophoretically coated on copper substrate at different voltages
Graphene/CNT mixture electrophoretically coated on copper substrate at different voltages
ACS Appl. Nano Mater. 2020, 3, 10, pp. 10003-10013
Metal recovery from lithium-ion batteries
 He developed the so-called MSX process: Dr. Bhave in his lab at Oak Ridge LaboratoryAtOak Ridge National Laboratory, USA, Dr. Ramesh Bhave's research group has developed a membrane-based solvent extraction process to recover cobalt, nickel, lithium and manganese. The so-called MSX process runs in a closed circuit and has been licensed to Momentum Technologies, Inc. in Dallas. Momentum's business focuses on the extraction of critical metals from e-waste.
He developed the so-called MSX process: Dr. Bhave in his lab at Oak Ridge LaboratoryAtOak Ridge National Laboratory, USA, Dr. Ramesh Bhave's research group has developed a membrane-based solvent extraction process to recover cobalt, nickel, lithium and manganese. The so-called MSX process runs in a closed circuit and has been licensed to Momentum Technologies, Inc. in Dallas. Momentum's business focuses on the extraction of critical metals from e-waste.
The metals are obtained in high-purity form and can be reused for the same purpose. Extraction takes place after the separation of recyclable plastics and metals. To reduce logistics costs, the plants are installed where the e-waste is available. The energy, labor and solvent requirements of the MSX process are low.
https://www.eurekalert.org/pub_releases/2020-10/drnl-ptr101420.php
Address of the author
Dr. Nagaraj N. Rao, RRR House, RRR Labs Pvt. Ltd, Plot 80, Sector 23, Navi Mumbai - 400 705 India; e-mail:


