- Part 2 - Results, discussion and summary / continued from Galvanotechnik 2/2024
Sol-gel coatings are typically limited in thickness in order to be able to cure without cracking. Curing with atmospheric pressure plasmas shifts the usable coating thickness range significantly upwards. This study combines curing with plasma jets with simultaneous functionalization of the coatings to achieve antimicrobial properties using a thermal and UV-curing sol-gel system. The infection load can be massively reduced by means of integrated copper particles.
(Ü1)Results and discussion
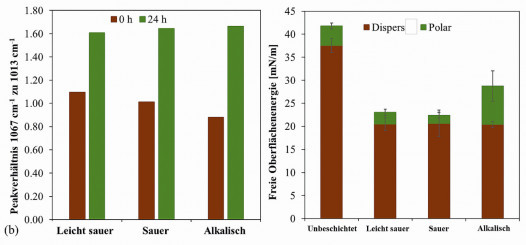
FTIR spectroscopy can be used to determine the progress of the reaction and the degree of polycondensation in the layer at different times after plasma curing (Fig. 2a). This makes it possible to describe chemical reactions and show their effects on the curing behavior. The primary reaction is described by the polycondensation of the Si-ethoxy groups (1067 cm-1) with each other to form a Si-O-Si network (1013 cm-1). There is a shift from the peak at 1067 cm-1 to the Si-O-Si peak at 1039 cm-1 and finally to the Si-O-Si peak at 1013 cm-1 with increasing aging time. The lack of recognizable differences between the individual spectra, apart from this shift, indicates that the chemical reaction taking place is limited to polycondensation. The degree of cross-linking was estimated as the ratio between the two prominent peaks at 1067 cm-1 and 1013 cm-1 (Fig. 2b). The higher this value is, the more complete the reaction was. Immediately after plasma curing, slight differences were detectable: the sol-gel systems catalyzed at low pH were cured better than the primary system. However, this difference was no longer present after 24 hours.
The variation of the catalyst method (i.e. the pH value) also influences the wetting behavior. While the standard sol-gel system (pH ~5) achieves an H2Ocontact angle of 95-97°, lowering the pH by adding an acid (1 M HCl) leads to increased contact angle (98-101°) with a decrease in the polar part of the surface free energy (Fig. 2c). This increase is even stronger for basic catalyzed sol-gel systems (pH ~ 13) by adding 1 M NaOH: without the influence of temperature or radiation, the system reacts within 5 minutes: the plasma intensified this cross-linking. The contact angle decreases to 80-82°.
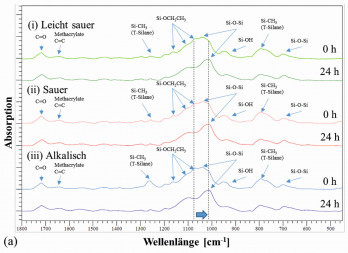 Fig. 2: (a) FTIR-ATR analysis of the brines used: superimposed spectra of the (i) slightly acidic sol-gel (pH ~ 5), (ii) acidic sol-gel (pH ~ 3) and (iii) alkaline sol-gel (pH ~ 13) immediately after coating and curing (0 h) and 24 h after curing. The samples were stored under normal room conditions, without exposure to UV or heat.
Fig. 2: (a) FTIR-ATR analysis of the brines used: superimposed spectra of the (i) slightly acidic sol-gel (pH ~ 5), (ii) acidic sol-gel (pH ~ 3) and (iii) alkaline sol-gel (pH ~ 13) immediately after coating and curing (0 h) and 24 h after curing. The samples were stored under normal room conditions, without exposure to UV or heat.
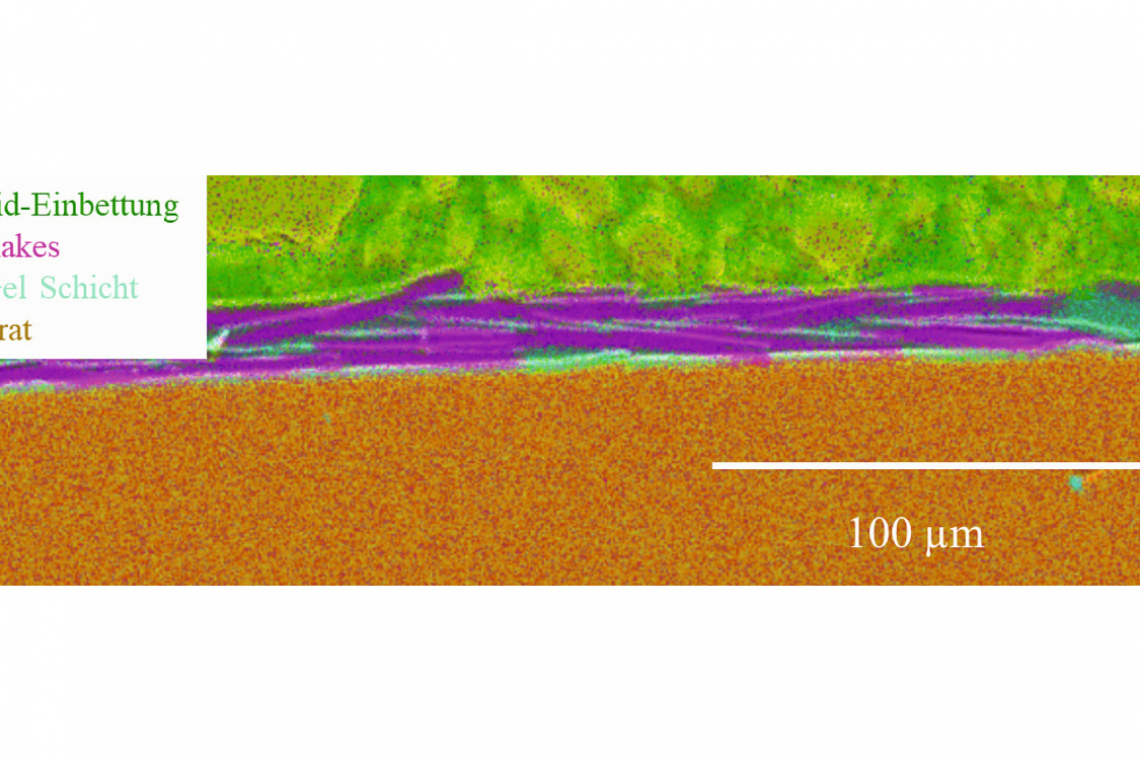
The water contact angle was further increased by introducing Cu particles and Cu flakes into the layer. The contact angle reached a maximum value of approx. 115° due to increased surface topography (roughness). Especially with the antimicrobial effect of copper based on direct surface contact to e.g. bacteria, the investigation of the morphology of the functionalization is essential. In order to investigate the penetration depth of the Cu particles into the still wet, pre-applied sol-gel layer during simultaneous plasma curing by the radiation from the plasma, investigations were carried out on the cross-sectional area of the coatings (i.e. the sol-gel type with pH 7). This showed a significant correlation between the plasma curing parameters and the particle penetration depth: Higher current in the discharge for plasma formation (i.e. higher power of the plasma jet) leads to higher energy in the plasma, but also to a higher gas and thus particle velocity [St7] and thus deeper penetration into the "wet" sol-gel layer (Fig. 3a). Reduced current reduces the penetration depth and the particles lie almost predominantly on the surface of the sol-gel coating (Fig. 3b). A similar result was observed if the sol-gel layer was already excessively cured or cross-linked before the particles were applied, which prevents the particles from penetrating. Re-polishing can "expose" the particles again (Fig. 3c).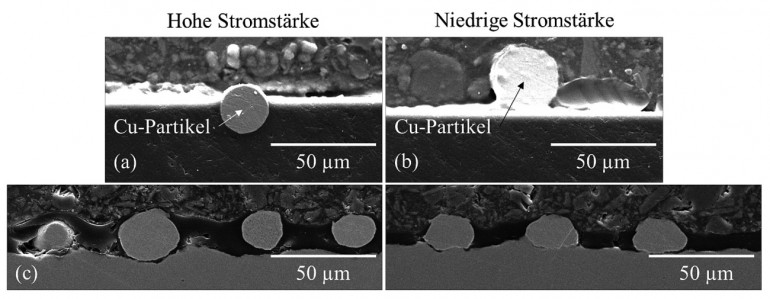 Fig. 3: SEM analysis on metallographically prepared cross-sectional surfaces for the penetration depth of copper particles into the still moist sol-gel layer.depending on the plasma parameters: (a) high penetration depth at high current intensity (180 A) and (b) low penetration depth at low current intensity (120 A). (c) Previously deeply implanted particles exposed by mechanical polishing
Fig. 3: SEM analysis on metallographically prepared cross-sectional surfaces for the penetration depth of copper particles into the still moist sol-gel layer.depending on the plasma parameters: (a) high penetration depth at high current intensity (180 A) and (b) low penetration depth at low current intensity (120 A). (c) Previously deeply implanted particles exposed by mechanical polishing
The antibacterial results of the coatings with superficially present copper are promising (see below), but corrosive attack of the particles is already evident during these tests. This does not manifest itself in the form of surface corrosion, but in the detachment of particles, which creates "holes" in the coating. This detachment of particles - as well as the antimicrobial effect - is absent when the sol-gel completely encloses the powder. However, the release of particles is achieved again by polishing the surfaces.
The embedding strategy was therefore changed. Cu flakes with a flat shape (approx. 34 µm diameter) were automatically and homogeneously scattered into the moist sol-gel via the conveyor gas flow and the plasma nozzle without using plasma excitation. Plasma curing without particles only took place in the subsequent step. The aim was to keep the Cu flakes on the surface due to their shape (no "sinking"), which was also partially achieved. The flakes were stacked so that they could partially reach the surface of the layer, but were still surrounded by sol-gel. At the same time, the sol-gel could still be cured similarly well in the plasma. Adhesion and corrosion problems no longer occurred.
The antimicrobial effect was tested for pre-painted wood veneers functionalized with the sol-gel systems. An antibacterial effect can be assumed if there is a reduction of bacteria (colony forming units, CFU) by 3 log levels (reduction of the infection load by 99.9 %) [Vi0, Jo0]. If copper is present on the surface of the samples (i.e. as freely dispersed or ground particles [1] or as flakes), the effect is good after 3 hours against the Staphylococcus aureus (S. aureus) species shown(Fig. 4a for spherical particles). Tests on sol-gel coatings even without the addition of Cu show that these particles are essential for achieving the antimicrobial effect: Surfaces without Cu particles showed a reduction of Staphylococcus aureus (S. aureus) by only 10 % after 30 minutes and about 62 % after three hours, with Cu addition by 5 log levels (99.999 %). The slight decrease without copper may be due to a lack of nutrients and liquid or to the mechanical effect of the rough surface, which can lead to irreversible destruction of the bacterium.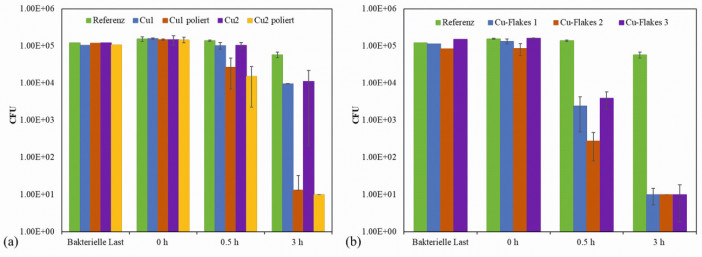 Fig. 4: Results of antibacterial tests with S. aureus on the substrates coated with sol-gel and (a) microparticles (without/with polishing and 180 A (1) and 120 A (2)) or (b) Cu flakes (1-3 refer to different plasma curing speeds (50 mm/s, 75 mm/s and 100 mm/s)) compared to (b) a reference sample at three time points (0, 0.5 and 3 hours)
Fig. 4: Results of antibacterial tests with S. aureus on the substrates coated with sol-gel and (a) microparticles (without/with polishing and 180 A (1) and 120 A (2)) or (b) Cu flakes (1-3 refer to different plasma curing speeds (50 mm/s, 75 mm/s and 100 mm/s)) compared to (b) a reference sample at three time points (0, 0.5 and 3 hours)
The flat Cu flakes used instead of the "spherical" Cu particles accelerate the antibacterial effect and do not require repolishing, as Figure 4b shows. The lack of complete sinking and the formation of "stacks" of flakes lead to a higher density of flakes and thus a higher copper concentration on the surface. In addition, the plasma parameters were also adjusted. After 0.5 h, a degradation of over 98 % was achieved for almost all three parameters (different speed of the plasma jet during curing, whereby higher speed reduces the interaction time or the applied plasma power in linear dependence), which is necessary to speak of an antibacterial effect. In comparison, the samples with the "spherical" particles were between 25 and 65 % after 0.5 h without post-treatment and below 90 % with post-treatment.
Summary
This study demonstrated the use of an atmospheric pressure plasma jet system to improve the application, functionalization and curing process of a thermal and UV curing sol-gel system. The plasma enables rapid curing, while UV radiation and heat accelerate the curing of thick sol-gel layers more efficiently than current methods. The degree of cross-linking and chemical reactions over time can be determined by FTIR spectra analysis. The Si-O-Si bond effectively describes the built-up Si network. pH changes can also be used to vary the surface free energy. Plasma curing enables precise and rapid modification of layer properties, including the integration of copper particles or flakes to improve antimicrobial properties (i.e. combination with plasma spraying). By applying round copper particles, the infection load can be reduced by 99.99% within 3 hours (90% after 30 minutes), while Cu flakes have an additional accelerating effect and enable a reduction of 98% to 99% in 30 minutes and of 99.99% within 3 hours.
Acknowledgements:
Financial support was provided by the Federal Ministry of the Republic of Austria for Climate Protection, Environment, Energy, Mobility, Innovation and Technology (BMK) and the Austrian Research Promotion Agency (FFG) through its funding program "Production of the Future" and the projects " "safeTOUCH" (project no. 881059) and "LiBio" (project no. 874472) as well as by the Federal Ministry of Agriculture, Forestry, Regions and Tourism with the funding program "Think.Wood" and the project "softTOUCHwood" (project no. 999891637).
Literature
[1] Chwatal, S.; Stummer, M.; Steiner, et al.: Thin Solid Film. (2022), 763, 139598.
[2] Even, A.; Vignaud, G.; Guitter, N. et al.: J. Coat. Technol. res. (2020), 17, 333-343.
[3] Fedel, M.; Poelman, M.; Zago, M. et al: Surf. Coat. Technol. (2015), 274, 9-17.
[4] Fedel, M.: J. Coat. Technol. Res. (2017), 14, 425-435.
[5] Guo, L.; Feng, W.; Liu, X. et al: Mater. Lett. (2015), 160, 448-451.
[6] Han, Y.-H.; Taylor, A.; Mantle, M.D. et al: J. Non-Cryst. Solids (2007), 353, 313-320.
[7] Issa, A.A.; Luyt, A.S.: Polymers (2019), 11, 537.
[8] Jose, M.; Szymańska, K.; Szymański, K. et al: J. Environ. Chem. Eng. (2020), 8, 104550.
[9] Kim, S.W. Korean J. Chem. Eng. (2011), 28, 298-303.
[10] Majoul, N.; Aouida, S.; Bessaïs, B.: Appl. Surf. Sci. (2015), 331, 388-391.
[11] Nablo, B.J.; Rothrock, A.R.; Schoenfisch, M.H.: Biomaterials (2005), 26, 917-924.
[12] Orwat, K.; Bernard, P.; Wróblewski, S. et al: Maced. J. Chem. Eng. (2018), 37, 215-224.
[13] Sakka, S. Sol-Gel Process and Applications. In Handbook of Advanced Ceramics; Elsevier: Amsterdam, The Netherlands, (2013); pp 883-910.
[14] Stummer, M., Stögmüller, P., Eichinger, T., et al.: Materials Science Forum (2017) Vol. 879. 1870-1875
[15] Toide, T.; Rosero-Navarro, N.C.; Miura, A. et al: J. Sol-Gel Sci. Technol. (2022), 104, 478-483.
[16] Videira-Quintela, D.; Guillén, F.; Montalvo, G. et al: Colloids Surf. B Biointerfaces (2020), 195, 111216.
[17] Wouters, M.; Wolfs, D.P.; van der Linde, M.C. et al: Prog. Org. Coat. (2004), 51, 312-319.