Aluminum components have been successfully treated with chromium(III)-containing passivations for years. A robust process with good reproducibility is required in production. To achieve this, it is necessary to understand and take into account the entire process from surface preparation in degreasing, pickling and decapping to passivation and drying.
The process is established and has found its way into various standards, but at the same time has to meet ever higher quality requirements. Especially in the case of the corrosion-prone and high-alloy aluminum alloy EN AW-2024 T3, it becomes clear what role the individual steps of the coating process play and that the application parameters must be precisely coordinated in order to achieve a satisfactory end result tailored to the high demands. This article takes a holistic view of the coating process. The main tasks of the individual process steps are described and the critical application parameters that are important for good corrosion protection are defined.
Introduction and definition
 Tab. 1: Typical coating process for chromium(III)-containing passivation of aluminumAlternativeprocesses based on trivalent chromium compounds (CrIII) have become established in surface technologyfor thepassivation of aluminum surfaces. They meet the requirements of RoHS, ELV and WEEE and REACh [1-3] and both the process solution and the layer produced on the aluminum surface are free of hexavalent chromium compounds [4]. The processes and the resulting layer can be described as chromium(III)-containing passivation. In addition, the processes and their intended use can be further described with the help of recognized standards.
Tab. 1: Typical coating process for chromium(III)-containing passivation of aluminumAlternativeprocesses based on trivalent chromium compounds (CrIII) have become established in surface technologyfor thepassivation of aluminum surfaces. They meet the requirements of RoHS, ELV and WEEE and REACh [1-3] and both the process solution and the layer produced on the aluminum surface are free of hexavalent chromium compounds [4]. The processes and the resulting layer can be described as chromium(III)-containing passivation. In addition, the processes and their intended use can be further described with the help of recognized standards.
The well-known SurTec 650 process, for example, is approved in accordance with MIL-DTL-81706B and parts can be coated and specified in accordance with MIL-DTL-5541F. SurTec 650 is a TYPE II coating, i.e. chromate-free, and can be used as CLASS 1A for bare corrosion protection and CLASS 3 for low electrical contact resistance [5].
The drawing in accordance with DIN 50935-2, Chromium(VI)-free passivation of non-ferrous metals - Part 2 is also possible. Aluminum surfaces coated with SurTec 650 correspond to "Coating type A - chromium(III)-containing passivation". This standard can also be used to define additional areas of application, such as the alloy composition of the aluminum, or the task of the passivation layer, such as bright corrosion protection, or the preparation for subsequent painting [6].
Chromium(III)-containing passivation has therefore been introduced to the market and specified in standardization. However, experts know that good and specified passivation alone is not enough to achieve a good protective effect. Passivation is interlinked with upstream and, in some cases, downstream process steps. Optimal properties of the manufactured part are only achieved when all these steps are optimized and coordinated.
Using the example of the alloy EN AW-2024 T3, it will be shown below that the overall process must be considered in order to achieve a good result. The tasks of the individual process steps and which application parameters are important for a satisfactory result are shown.
Coating process using the example of the aluminum alloy EN AW-2024 T3
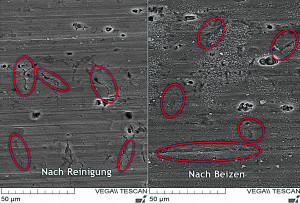 Fig. 2: Left: After cleaning, narrow gaps and cracks can be seen in the surface. Right: After pickling, the cracks are widened. The influence of pickling on the achievable corrosion protection can be measuredForgood coating quality, it is not only the passivation alone that is decisive, but also the upstream process steps. The overall process must therefore be coordinated.
Fig. 2: Left: After cleaning, narrow gaps and cracks can be seen in the surface. Right: After pickling, the cracks are widened. The influence of pickling on the achievable corrosion protection can be measuredForgood coating quality, it is not only the passivation alone that is decisive, but also the upstream process steps. The overall process must therefore be coordinated.
The aluminum surface must be clean, oxide-free and completely water-wettable in order to form a good passivation layer [7]. In the case of alloyed aluminum, it is also important that the material composition of the surface does not differ significantly from that in the material. For example, if there is a high heavy metal concentration in the alloy, as in the case of EN AW-2024 T3, care must be taken to ensure that as little of the heavy metal as possible is accumulated on the surface. Copper in particular must be avoided, as it is more noble than aluminum, can form electrochemical local elements and thus accelerate corrosion processes.
A typical coating process is described in Table 1 and consists of the following steps: degreasing, pickling (optional), decapping and passivation.
|
Process step |
Product |
Remark |
|
|
1 |
Cleaning |
SurTec 061 / 089 |
Removes oil, grease and other contaminants |
|
2 |
Rinsing |
Influenced by water quality and rinsing time |
|
|
3 |
Pickling |
SurTec 181 |
Removes corrosion products and deformed boundary layer |
|
4 |
Rinsing |
Influence of water quality and rinsing time |
|
|
5 |
Decapping |
SurTec 496 |
Removes pickling bast and heavy metals |
|
6 |
Rinsing |
Influence of water quality and rinsing time |
|
|
7 |
Passivation |
SurTec 650 |
Chromium(III)-containing passivation |
|
8 |
Rinsing |
Influence of water quality and rinsing time |
|
|
9 |
Drying |
RT hot air |
Influence of temperature and drying time |
Tasks of the upstream process steps Cleaning
The importance of the first process step, cleaning, is obvious. Contamination from process steps upstream of chemical surface treatment should be removed. If residues such as grease or oil remain on the surface and cover it, the passivation layer cannot be formed there later, resulting in an insufficient protective effect. There are various reports in the specialist literature that describe the process and its effect in detail [8].
Pickling
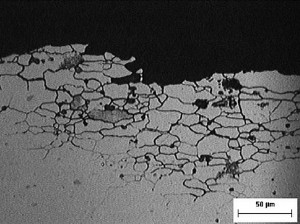
The task of pickling, on the other hand, is less obvious. First of all, the pickling process dissolves oxides and corrosion products that have formed on the surface of the base aluminum during storage. In addition, metallic aluminum is dissolved, the surface is chemically milled and structural defects are removed. In the case of high-alloy aluminum such as EN AW-2024 T3, for example, grain boundary corrosion can form. In these fine cracks, which can reach deep into the material (Fig. 1), passivation cannot form later and foreign salts, which trigger corrosion, are easily trapped.
Cavities can also form as a result of mechanical impact, for example during rolling. If the surface is viewed at high magnification using scanning electron microscopy, very fine, crack-like structures can be seen after cleaning (Fig. 2). The cracks are widened by pickling. These wider cavities can then be rinsed out more easily, the passivation electrolyte can penetrate into these cavities and passivate the surface without defects.
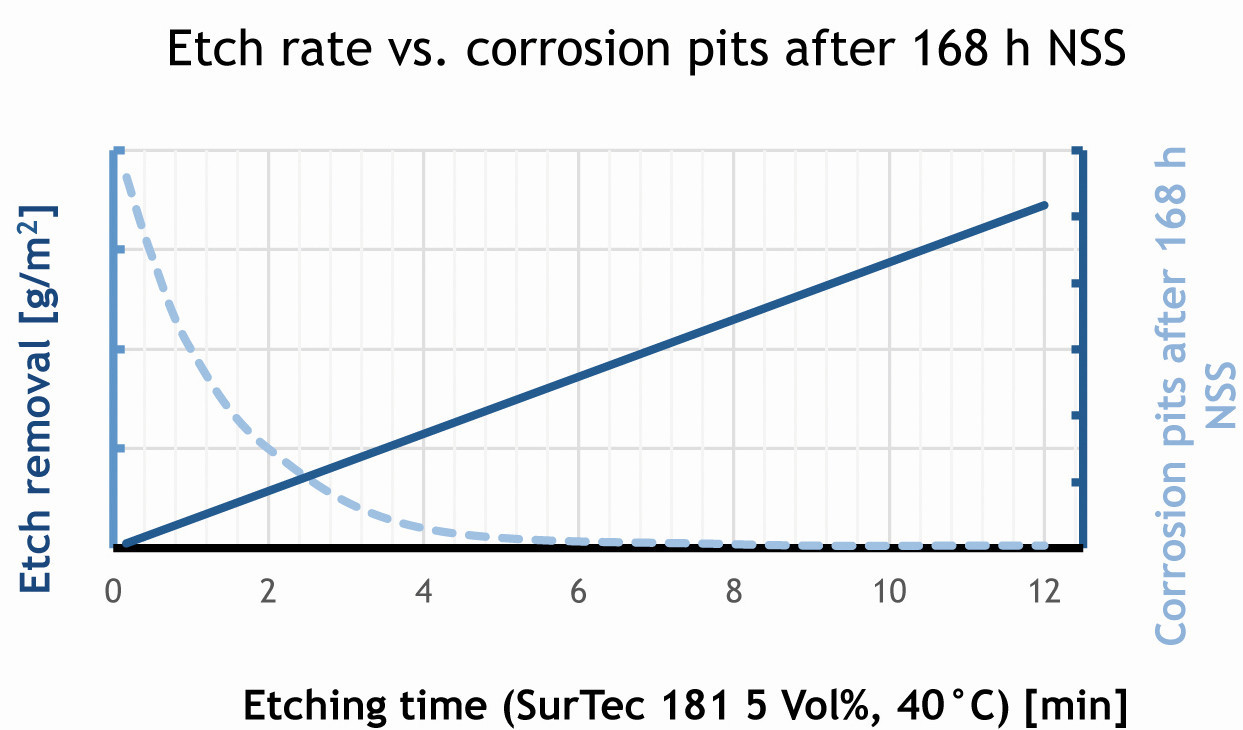 Fig. 3: The longer the pickling time, the higher the pickling removal and the lower the number of corrosion points
Fig. 3: The longer the pickling time, the higher the pickling removal and the lower the number of corrosion points
Figure 3 graphically illustrates the dependency of pickling time and corrosion protection. Only after sufficient pickling time and thus sufficient material removal are disruptive defects removed and the corrosion protection becomes increasingly better.
Decapping
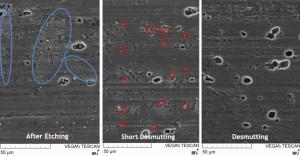
Aluminum is preferentially dissolved during the upstream alkaline pickling process. The heavy metals from the alloy accumulate on the surface and form the so-called pickling bast. Small inclusions with a very high copper concentration are also found in the alloy with a high copper content (Fig. 4). These approximately 10-20 µm copper phases form electrochemical local elements and trigger pitting corrosion (Fig. 5). During decapitation, the heavy metals of the aluminum alloy, which in the case of AW-2024 T3 are copper components in particular, are selectively removed.
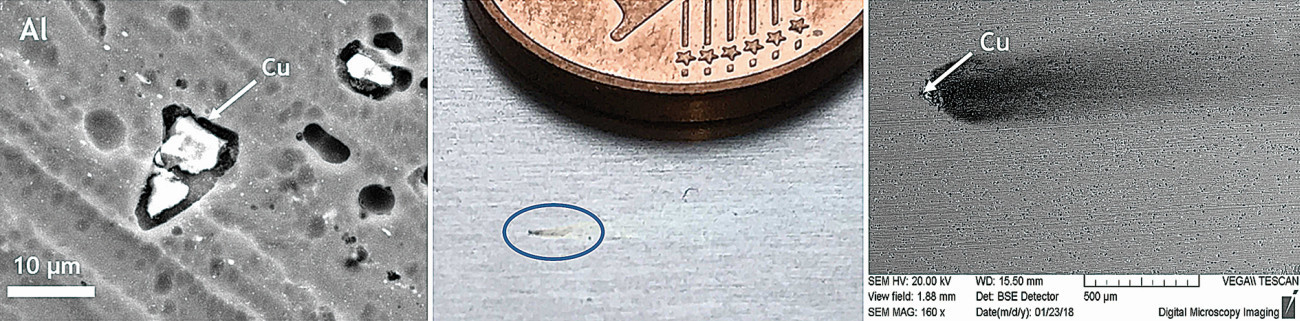 Fig. 4: Left: Copper phases in EN AW-2024 T3, where corrosion pits can form Center: Corrosion pit; Right: SEM analysis of a pit; a high Cu concentration can be measured in the center
Fig. 4: Left: Copper phases in EN AW-2024 T3, where corrosion pits can form Center: Corrosion pit; Right: SEM analysis of a pit; a high Cu concentration can be measured in the center
The effect of decapitation can again be seen using scanning electron microscopy. After the alkaline pickling process, the fine-grained and evenly distributed pickling paste is clearly visible. Even after a short treatment time of a few seconds, the pickling bast is removed and the bright copper phases are still clearly visible (Fig. 6). Only after a longer treatment time of several minutes are these phases also removed and the surface is ready for passivation.
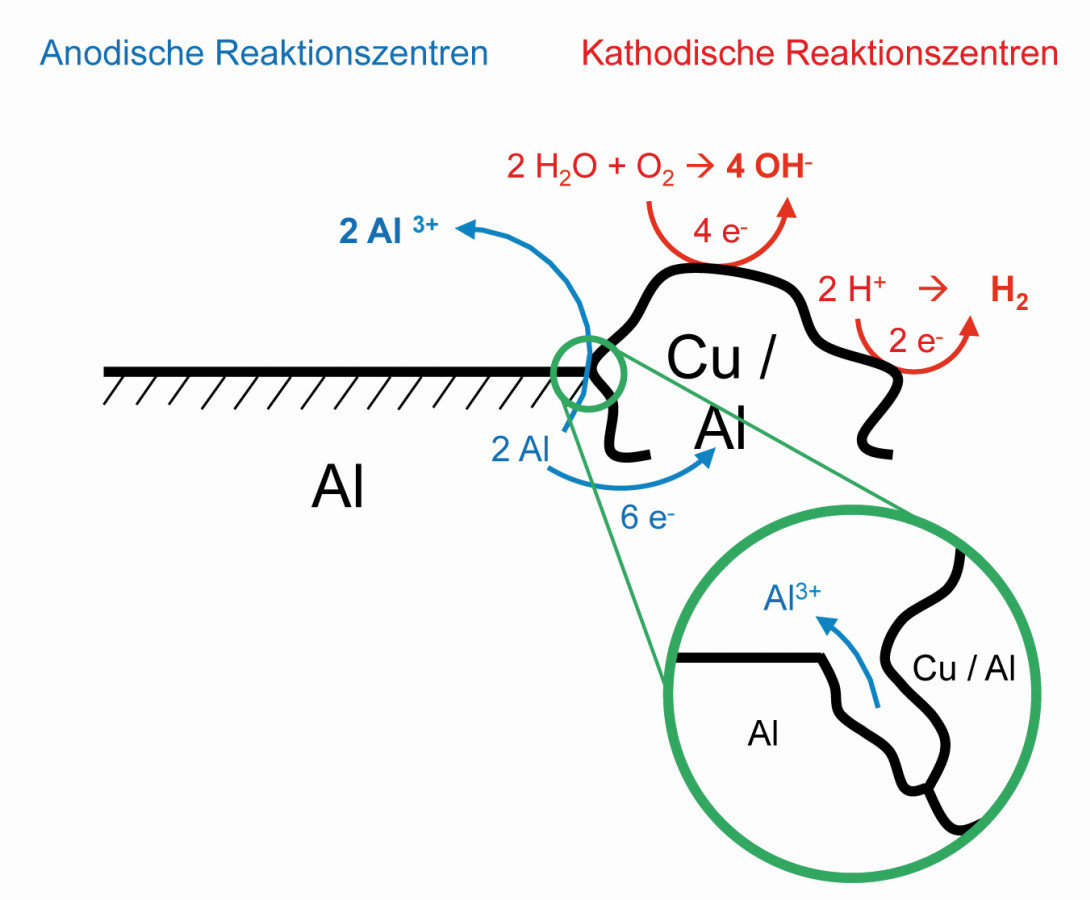 Fig. 5: Mechanism of pitting corrosion at the aluminum - copper phase boundary
Fig. 5: Mechanism of pitting corrosion at the aluminum - copper phase boundary
A long decapping time therefore reduces the heavy metal concentration on the surface and copper accumulations in particular are removed. Figure 7 shows the dependence of decap time and corrosion protection.
Passivation and its parameters
During passivation, mixed oxides of chromium(III), zirconium(IV) and aluminum(III) are deposited on the surface. This deposition is continuous and the longer the treatment, the thicker the passivation layer produced [10, 11].
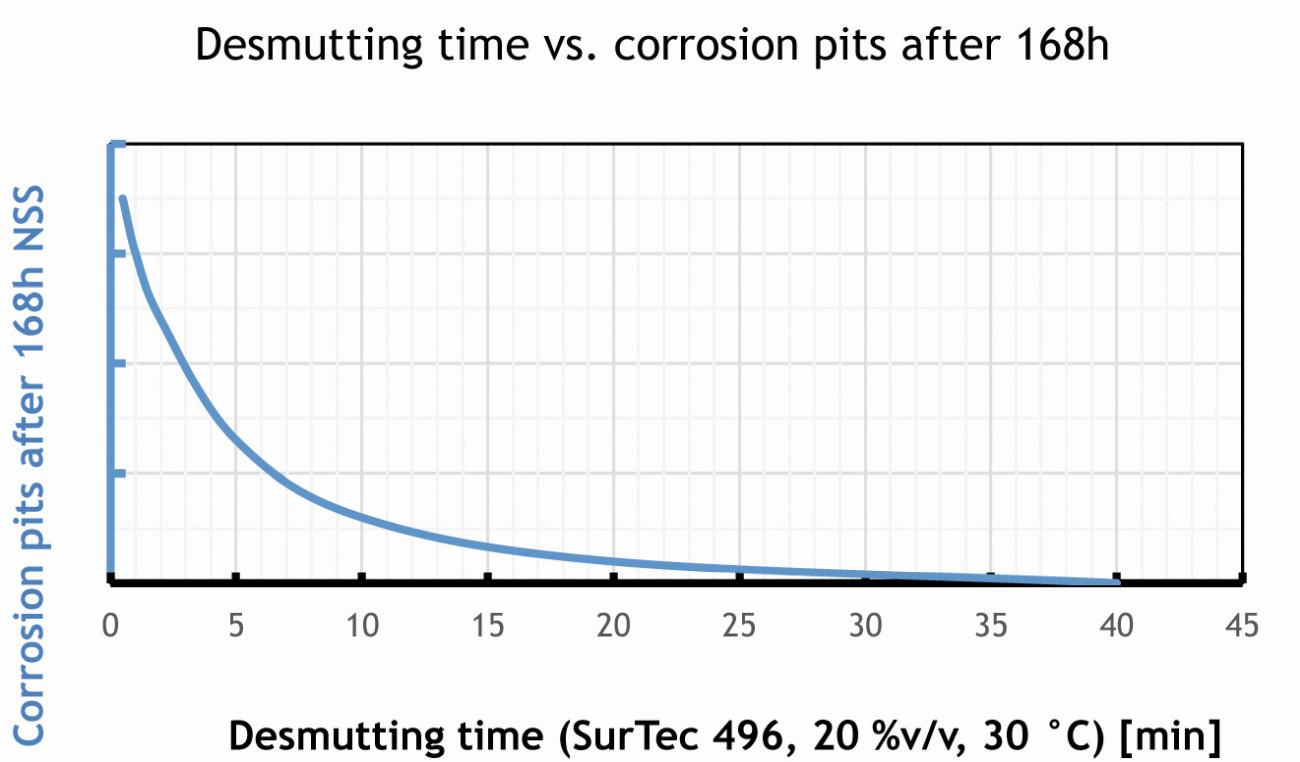 Fig. 7: In the case of EN-AW 2024 T3, a longer deposition time improves the corrosion protection
Fig. 7: In the case of EN-AW 2024 T3, a longer deposition time improves the corrosion protection
In principle, it could be assumed that a very thick layer also provides particularly good corrosion protection. However, as the coating thickness increases, stresses occur in the coating and fine cracks appear. In the case of over-passivation, which is also known to occur in processes containing chromate (yellow chromating) and must be avoided, the cracks become so strong that they represent defects where corrosion can occur more quickly locally. Electron micrographs show the formation of cracks as a function of the treatment time (Fig. 8).
 Fig. 9: Left: Cross-section with color etching according to Kroll. Right: Surface after long immersion time in SurTec 650 In the case of EN AW 2024 T3, it is noticeable that the material (and therefore also the surface) is inhomogeneous and consists of different alloy phases that are present next to each other. Figure 9 shows a cross-section with color etching according to Kroll, where the brown areas show the copper precipitates. Opposite is an SEM image of the surface after a long treatment time in SurTec 650 at the same magnification. It can be seen that the cracks occur preferentially at the copper precipitates.
Fig. 9: Left: Cross-section with color etching according to Kroll. Right: Surface after long immersion time in SurTec 650 In the case of EN AW 2024 T3, it is noticeable that the material (and therefore also the surface) is inhomogeneous and consists of different alloy phases that are present next to each other. Figure 9 shows a cross-section with color etching according to Kroll, where the brown areas show the copper precipitates. Opposite is an SEM image of the surface after a long treatment time in SurTec 650 at the same magnification. It can be seen that the cracks occur preferentially at the copper precipitates.
In the area of the grain boundaries, the passivation layer is created at different speeds and also has a slightly different composition [12]. This results in preferential stresses that lead to cracking during long treatment times. For the quality of the coating and the corrosion protection that can be achieved, this means that the treatment time must be precisely coordinated and the coating must be thick enough, but not too thick.
The user must therefore adhere very precisely to the treatment parameters such as time, temperature and pH value in order to achieve an optimum result. An additional problem is that different alloys show the effect described above to varying degrees. Some alloys react quickly with the passivation solution and the cracks appear correspondingly quickly. Other aluminum alloys are more reactive and require longer treatment times until the layer is sufficiently thick and shows a sufficient barrier effect against corrosive media. Compromises are therefore often made in production with different aluminum alloys, which increasingly reduces the optimum process window.
However, by adding surfactants to the passivation solution, it is possible to reduce the stresses in the resulting layer [13]. The surfactants reduce the surface tension of the passivation solution and improve the wetting of the aluminum surface, even in existing cracks and cavities. This results in a more uniform deposition and less tension in the layer. As a result, a wider process window is possible again.
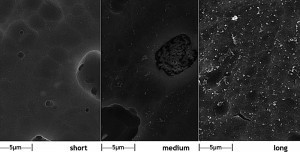 Fig. 10: SurTec 650 with SurTec 650 A surfactant; left: short passivation time, coating still too thin. Center: medium passivation time, optimal. Right: long passivation time, thick but almost defect-free layerTheeffect of a suitable surfactant mixture is shown in Figure 10. Even after long treatment times, the layer is almost free of cracks. Sufficiently thick layers without defects are produced over a wide time window, which significantly improves the robustness and repeatability of the process.
Fig. 10: SurTec 650 with SurTec 650 A surfactant; left: short passivation time, coating still too thin. Center: medium passivation time, optimal. Right: long passivation time, thick but almost defect-free layerTheeffect of a suitable surfactant mixture is shown in Figure 10. Even after long treatment times, the layer is almost free of cracks. Sufficiently thick layers without defects are produced over a wide time window, which significantly improves the robustness and repeatability of the process.
The surfactants enable a more uniform deposition of the passivation layer, but are not incorporated into the layer itself. Other measurable layer characteristics remain the same. For example, there is no difference in the coating composition measured by EDX. Similarly, the deposition rate and the resulting coating weight are not changed by the surfactants. In addition, there is no influence on the paintability or the surface tension of the passivation layer and the electrical contact resistance remains low, as required by MIL-DTL-5541 Type II Class 3.
The lower-stress layer has a very positive effect in the event of strong temperature changes, for example rapid drying at high temperatures. When drying after treatment, the surface must not normally be heated above 65 °C, as drying too quickly can increase the stress in the coating and the formation of cracks. For components with complex geometries, this temperature is often not sufficient to dry the surface within a practicable time. In order to accelerate the drying process, higher temperatures are therefore often required in industrial practice, while maintaining the same corrosion protection. Figure 11 shows aluminum sheets of alloy 6060 T6 treated with and without a surfactant mixture, dried at 80 °C after rinsing and then exposed to corrosion for 168 h in a neutral salt spray in accordance with ISO 9227. While slight discoloration and initial corrosion spots already appear during normal treatment in SurTec 650, the passive layer created more evenly by the surfactant mixture is still completely intact.
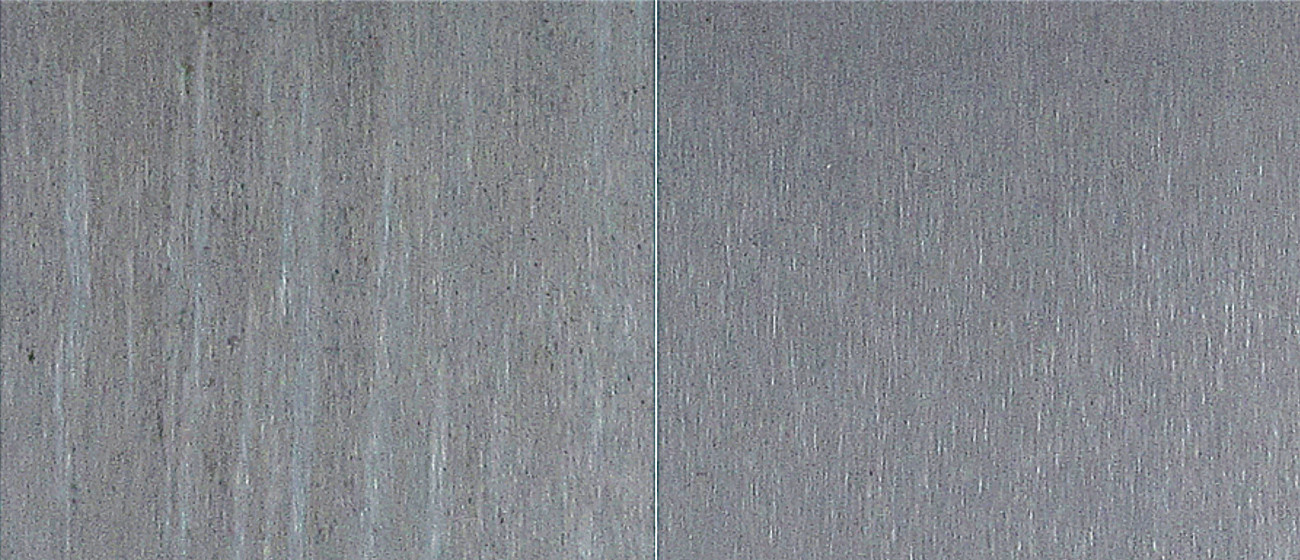 Fig. 11: EN-AW 6061 T6 dried at 80 °C, 20 min; left: without surfactant mixture, right: with SurTec 650 A, both after 168 h NSS
Fig. 11: EN-AW 6061 T6 dried at 80 °C, 20 min; left: without surfactant mixture, right: with SurTec 650 A, both after 168 h NSS
Summary
In order to achieve the best possible surface quality of chromium(III)-containing passivation, the overall process must be considered. Each individual process step must be tailored to the requirements and monitored.
During cleaning, all residues that would interfere with the subsequent process steps must be removed. The pickling process has the task of removing oxides and corrosion products, but mechanically deformed boundary layers and near-surface defects are also removed. For extruded and rolled semi-finished products, a longer pickling time can lead to better corrosion protection results. For alloys containing heavy metals, efficient decapitation is necessary to reduce the heavy metal concentration on the surface. If decapitation is able to selectively remove the heavy metal, longer treatment times can in turn lead to better corrosion protection results.
When passivating high-alloy aluminium, the passivation time is essential and must be kept within a narrow window. On the one hand, the passivation layer that forms should be thick enough to have a correspondingly good barrier effect, and on the other hand, over-passivation with defects in the layer must be avoided.
To optimize the process and improve process stability, a surfactant mixture was developed that can be added to the passivation bath. The surfactants lead to a more uniform coating formation without changing the coating properties. In particular, it should be emphasized that the surfactants widen the process window for optimum treatment. Fewer defects are formed and the more evenly deposited layer is more heat-resistant.
Literature
[1] Directive 2002/95/EC on the restriction of the use of certain hazardous substances in electrical and electronic equipment, European Parliament, European Council, January 27, 2003
[2] Directive 2000/53/EC on end-of-life vehicles, European Parliament, European Council, September 18, 2000
[3] REACh - Regulation of the EU, No. 348/2013 of the Commission of April 17, 2013
[4] Suib, S.L. et al.: Determination of Hexavalent Chromium in NAVAIR Trivalent Chromium Process (TCP) Coatings and Process Solutions, Metalfinishing (2009), No. 2
[5] Qualified Products Database, Department of Defense, United States of America, QPL-81706, https://qpldocs.dla.mil
[6] DIN 50935-2:2015-12, Chromium(VI)-free passivation of non-ferrous metals - Part 2: Aluminum and aluminum alloys, Beuth Verlag Berlin
[7] Aluminum pocket book, Aluminium-Verlag GmbH, 1974
[8] Grün, R.: Industrial parts cleaning: An overview, Galvanotechnik (2016), No. 10
[9] Internet research, https://www.researchgate.net/, 2020
[10] Volk, P.: Chromium(III)-containing passivation for aluminum, Jahrbuch Oberflächentechnik, Giesel Verlag, Vol. 57, 2006, pp. 273-280
[11] Volk, P.: Real alternative, metalloberfläche (2006), No. 5
[12] Honselmann, J.; Volk, P.: Mankel, E.: Analysis of the layer formation of chromium(III)-containing aluminum passivations, Galvanotechnik (2015), No. 4
[13] SurTec International GmbH, data sheet SurTec 650 A, www.surtec.com