A US patent from 1945 describes the use of mixtures of alkanesulfonic acids with 1 to 5 carbon atoms for the electrodeposition of lead and nickel on various metals, without giving details of the exact composition of the electrolytes and the electrolysis parameters [1]. From 1982 onwards, several patents described the use of alkanesulphonic acid electrolytes for the electrolytic tinning of steel in the production of tinplate as an alternative to the fluoroborate electrolytes used up to then [2-4]. From 2003, a new process made it possible to produce large quantities of methanesulfonic acid in the purity required for galvanic processes [5]. While the annual production capacity was 10,000 tons in 2003, it was increased to 30,000 tons in 2012 due to rising demand. A further increase in production capacity to 50,000 tons per year is planned for the end of 2021 [6]. A few months ago, BASF also increased its methanesulfonic acid capacity again with the commissioning of a new plant (Fig. 1)
 Fig. 1: The industrial use of methanesulfonic acid in industry has been increasing continuously since 2003. In addition to electroplating, it is used in biofuel synthesis and industrial cleaning. In Ludwigsburg, BASF recently increased capacity again with a new methanesulfonic acid plant . The following sections provide an overview of the use of methanesulfonic acid electrolytes for the deposition of metals and alloys in electroplating. Most publications and patents on the use of methanesulfonic acid in electroplating deal with the tinning of strip steel for the production of tinplate or the deposition of tin and tin alloys on copper to produce solderable surfaces. The acid is already being used in industry in these areas. There are also individual studies on the deposition of gold, silver, copper, indium and bismuth from methanesulfonic acid electrolytes. In addition to the use of methanesulfonic acid in electroplating baths, the acid is also used as a catalyst in environmentally friendly industrial and household cleaners and in the chemical industry.
Fig. 1: The industrial use of methanesulfonic acid in industry has been increasing continuously since 2003. In addition to electroplating, it is used in biofuel synthesis and industrial cleaning. In Ludwigsburg, BASF recently increased capacity again with a new methanesulfonic acid plant . The following sections provide an overview of the use of methanesulfonic acid electrolytes for the deposition of metals and alloys in electroplating. Most publications and patents on the use of methanesulfonic acid in electroplating deal with the tinning of strip steel for the production of tinplate or the deposition of tin and tin alloys on copper to produce solderable surfaces. The acid is already being used in industry in these areas. There are also individual studies on the deposition of gold, silver, copper, indium and bismuth from methanesulfonic acid electrolytes. In addition to the use of methanesulfonic acid in electroplating baths, the acid is also used as a catalyst in environmentally friendly industrial and household cleaners and in the chemical industry.
Properties of methanesulfonic acid
Methanesulfonic acid differs from sulfuric acid in that one OH group of sulfuric acid is replaced by a CH3 group (Fig. 2). Due to their similar structure, many properties of both acids are also similar [5, 7].
In contrast to dibasic sulphuric acid, methanesulphonic acid is a monobasic strong organic acid (pKs = -1.9), which is almost completely dissociated in a 0.1 molar solution. It is thermally stable up to a temperature of 180 °C and chemically and electrochemically stable in the potential range from -1.4 V to +2.4 V. It is therefore not affected by the addition of strong oxidizing agents such as hydrogen peroxide or by electrolysis in which oxygen is generated at insoluble anodes. It has a low vapor pressure, is colorless, odorless and does not form toxic gases. Compared to sulphuric acid or nitric acid electrolytes, its scattering and covering properties are better, so that smooth, high-purity copper cathodes can be deposited in compact form from methanesulphonic acid electrolytes without the addition of additives and silver [8, 9].
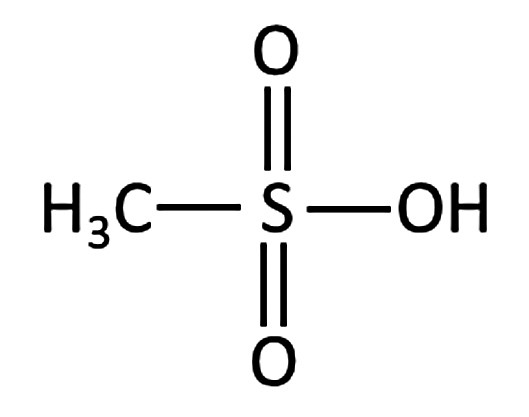 Fig. 2: Structural formula of methanesulfonic acid
Fig. 2: Structural formula of methanesulfonic acid
An essential prerequisite for electrolytic metal deposition is a high solubility of the metals in the electrolyte. As Table 1 shows, many metal sulphonates are easily soluble in water.
Lead and silver in particular, whose sulphates are poorly soluble, have a very high solubility in methanesulphonic acid electrolytes. The solubility of tin, zinc, nickel, iron, copper, cobalt and cadmium is also considerably higher compared to sulphuric acid electrolytes. Due to the high solubility for many metals, the very good chemical and electrochemical stability, the good electrical conductivity and the low toxicity, methanesulfonic acid electrolytes are suitable for use in electroplating and for the electrolytic extraction and refining of metals.
One disadvantage of methanesulfonic acid is its higher price compared to sulfuric acid. Furthermore, calcium methanesulfonate is easily soluble, so that methanesulfonate cannot be separated by precipitation with milk of lime in wastewater treatment. Methanesulfonic acid is easily biodegradable and decomposes into carbon dioxide and sulfate. It is therefore part of the natural sulphur cycle and is also known as "green acid".
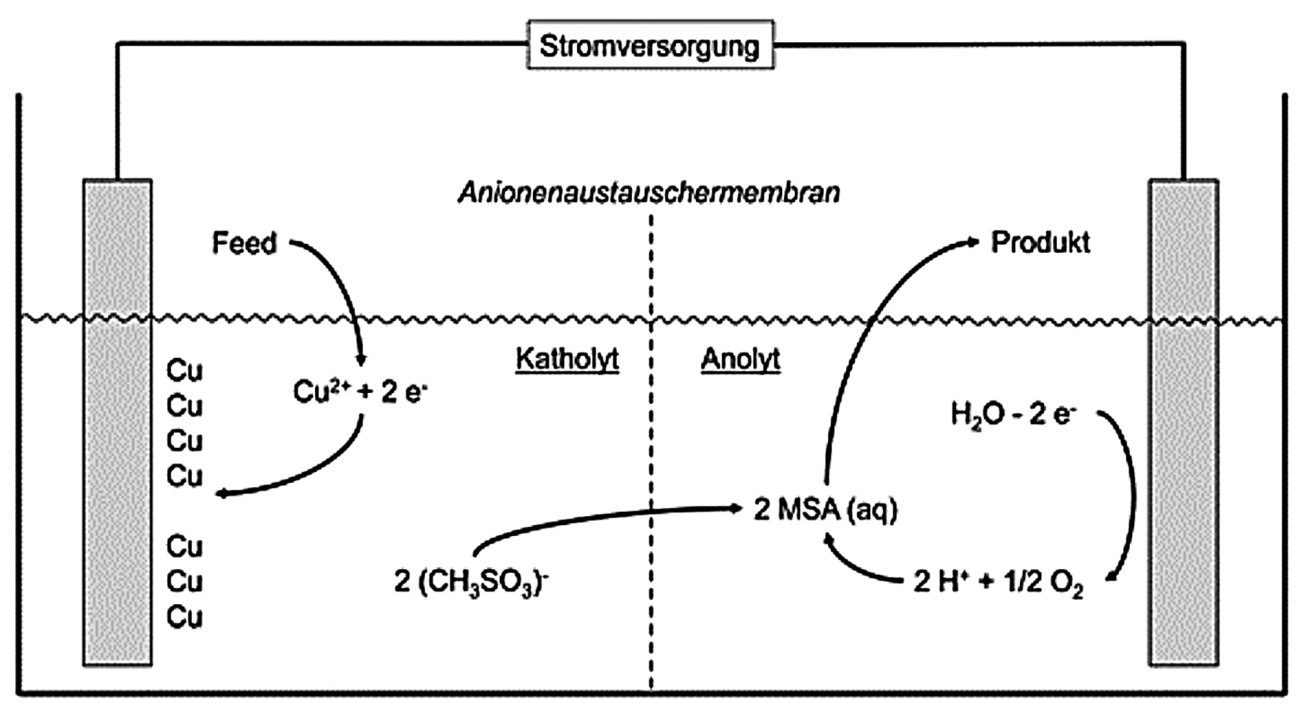 Fig. 3: Electrolytic recovery of methanesulfonic acid
Fig. 3: Electrolytic recovery of methanesulfonic acid
Due to the higher price of methanesulfonic acid, the electrolytic recovery of methanesulfonic acid in an electrolysis cell, in which the cathode chamber and anode chamber are separated by an anion exchange membrane, is a good option [7]. Figure 3 shows an example of this process for the treatment of copper-containing electrolytes. The electrolyte is fed into the cathode chamber where the copper is cathodically deposited. The copper can either be sold or remelted into new anodes. The methane sulphonate ions enter the anode chamber via the ion exchange membrane and can thus be fed back into the process. The impurities contained in the electrolyte (e.g. decomposition products of the additives) accumulate in the cathode chamber and can be disposed of via the waste water treatment system if the limit values defined by the process are exceeded after the copper has been removed as far as possible.
Separation of tin and tin alloys
Deposition of tin
Electroplated tin layers are of great industrial importance as they protect the base metal from corrosion and oxidation by air and have good solderability. Major applications include the tinning of strip steel (tinplate), wires and printed circuit boards for the electrical and electronics industry. In addition to pure tin, various alloys of tin with other metals such as lead, copper, zinc, silver, nickel and cobalt are also used.
In principle, tin can be electrolytically deposited from acidic, neutral or alkaline electrolytes. In addition to electroplating, sulphuric acid tin electrolytes are also used in refining electrolysis to produce high-purity tin. However, due to the risk of anode passivation, the sulphuric acid electrolyte is not suitable for high current densities. Another problem with this electrolyte is the oxidation of bivalent tin to tetravalent tin and the associated formation of colloidal or gel-like tin acids, which are difficult to filter.
In the past, electrolytes based on fluoroborate were often used for the industrial deposition of tin and tin-lead alloys at high current densities. The advantages of this electrolyte are the good solubility of tin and lead, good throwing power, the use even at high current densities and thus the realization of high deposition rates, high current yields and the deposition of fine-grained layers. Fluoroborate electrolytes have relatively good chemical stability. However, decomposition occurs to a small extent with the formation of hydrofluoric acid:
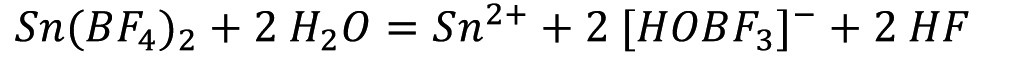
<1>
As a result, this electrolyte is highly corrosive and there is a risk of toxic hydrogen fluoride being emitted. Another disadvantage is the very complex waste water treatment. Compared to tetrafluoroboric acid, methanesulfonic acid is significantly less corrosive and has slightly reducing properties, meaning that the risk of the formation of tetravalent tin and thus sludge formation is somewhat lower. It is also less toxic. The LD50 value for tetrafluoroboric acid is 495 mg/kg and for methanesulfonic acid 1158 mg/kg. Methanesulfonic acid electrolytes therefore have clear advantages over tetrafluoroboric acid or sulfuric acid electrolytes for the electrodeposition of tin or tin-lead alloys. For this reason, systematic investigations into electrolytic tinning from methanesulfonic acid electrolytes have been carried out since around 1985, particularly for the tinning of strip steel and copper.
The annual global production capacity for tinned sheet steel (tinplate) is over 10 million tons. Tinplate is mainly used in the form of cans and tins for food/pet food (approx. 45%), chemical/technical products (approx. 21%), beverages (approx. 16%) and closures (approx. 18%). The process steps of cold rolling, degreasing, annealing and rerolling take place before electrolytic tinning. After electrolytic tinning, the tin layer can be inductively melted, the surface passivated to prevent the formation of tin oxides, painted or foil laminated. The strip tinning lines usually work with current densities between 1000 and 6000 A/m2 and strip speeds of up to 600 m/min, whereby the steel strip can be tinned on one or two sides. Depending on customer requirements, the tin coating is 1 to 15 g/m2, which corresponds to layer thicknesses of approx. 0.1 to 1.5 µm. The tin coating on tinplate must meet very high quality requirements. It must completely cover the base material, otherwise corrosion may occur. Special requirements are also placed on the adhesion of the tin layer to the base material, as forming into cans or tins takes place after tinning. During the forming process, the tin layer also serves as a lubricant. In the case of painted tinplate, the wetting behavior and paint adhesion are of great importance and in the case of unpainted tinplate, the visual appearance, in particular the gloss, is of great importance.
The additives used for strip steel tinning in tetrafluoroboric acid electrolytes do not work optimally in the methanesulfonic acid electrolyte. It was therefore necessary to search for and develop new, highly effective additive systems for tin deposition.
The additive systems required for tin deposition usually consist of surface-active substances, grain refiners, primary and secondary brighteners and oxidation stabilizers [10]. The patent literature [3, 11-15] in particular describes various additive systems for strip steel tinning at high current densities, the complete description of which is beyond the scope of this publication. Patent DE3902043 [15], for example, describes an electrolyte with 20 g/L tin, 70 to 100 g/L methanesulfonic acid and an additive system consisting of a nonyphenol polyglycol ether with 15 mol ethylene oxide, an aldol condensation product with acylic ketones and methanal for bright tin plating at high current densities. In her dissertation, Wehner [16] investigated the effect of various non-ionic polyethoxylated surfactants such as a propylene-ethylene oxide polymer (Pluronic RPE 310), naphthalene ethoxylates (Lugalvan), various nonylphenol ethoxylates (Lutensol AP) and polyeoxyethylene laryl ethers on the deposition of tin and tin-silver alloys from methanesulfonic acid electrolytes.
Although methanesulfonic acid has a weak reducing effect, some of the divalent tin is oxidized to the tetravalent stage by the introduction of oxygen from the air into the electrolyte. In slow processes, precipitates of basic tin oxides are formed, which are difficult to filter and are partially incorporated into the cathodically deposited tin layer. Particularly in strip steel tinning, the high strip speeds lead to an increased entry of air into the electrolyte. The main oxidation inhibitors used in methanesulfonic acid electrolytes are cresolsulfonic acid, hydrazine, ascorbic acid and hydroquinone and its derivatives.
Due to their advantages over tetrafluoroboric acid electrolytes, methanesulfonic acid electrolytes are now used in electrolytic tinning by some tinplate producers. In addition to advantages in the technological process and in waste water treatment, the quality of the tinplate has also been further improved as a result.
As a rule, the tin anodes used in electrolytic tinplate production contain low levels of lead. Due to the almost identical standard electrode potentials for tin and lead (see next chapter), the lead dissolves anodically with the tin, accumulates in the electrolyte and is deposited cathodically with the tin. Especially when using tinplate for food packaging, the lead concentration in the tin layer must not exceed 100 µg/g. For this reason, up to 5 g/L sulphate is added to the methanesulphonic acid electrolyte to separate lead as sparingly soluble PbSO4. Martyak and Seefeldt [17] determined that sulphate has only a minor influence on the quality of the tin layer.
In addition to the deposition of tin on steel, studies were also carried out on the deposition on copper. According to Rosenstein [18], the optimum electrolyte composition for tin deposition on copper is 200 to 250 g/L methanesulfonic acid, 35 to 55 g/L tin and an electrolyte temperature of 21 to 54 °C.
In addition to electroplating, methanesulfonic acid electrolytes are also used industrially today in tin refining electrolysis.
|
Metal ion |
Methane sulphonate (g/L) |
Sulphate (g/L) |
|
Ag+ |
713 |
9 |
|
Ca2+ |
656 |
3 |
|
Cd2+ |
975 |
646 |
|
Co2+ |
630 |
335 |
|
Cu2+ |
546 |
215 |
|
Fe2+ |
690 |
266 |
|
Fe3+ |
502 |
440 |
|
Hg2+ |
707 |
0 |
|
Mg2+ |
378 |
317 |
|
Mn2+ |
849 |
532 |
|
Ni2+ |
665 |
378 |
|
Pb2+ |
1075 |
0 |
|
Sn2+ |
1066 |
305 |
|
Zn2+ |
792 |
536 |
Deposition of tin-lead alloys
Coatings made of tin-lead alloys are widely used as solder materials due to their low melting point, good mechanical properties and technical reliability. Tin and lead are miscible over the entire concentration range and do not form intermetallic compounds. The eutectic alloy with 63 % Sn and 37 % Pb and a melting point of 183 °C is of particular interest as a solder material.
For electrolytic alloy deposition, it is of great advantage that Sn and lead have almost the same standard electrode potentials:
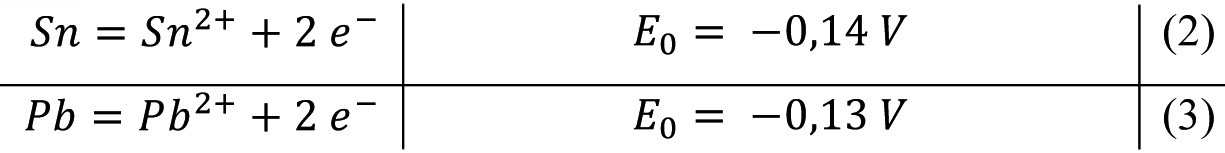
As the current density-potential curves for the cathodic deposition of tin and lead are almost identical in additive-free electrolytes, the deposition of tin-lead alloys is relatively straightforward over the entire concentration range. The desired alloy composition is set via the concentration ratio of tin and lead in the electrolyte [14, 17]. According to Rosenstein [17], the optimum electrolyte composition for the deposition of an alloy with 60 % Sn and 40 % Pb is 16 g/L Sn and 10 g/L Pb and for an alloy with 90 % Sn and 10 % Pb 21 g/L Sn and 3 g/L Pb.
According to various patents, the same additives can be used for the electrodeposition of tin, tin-lead alloys and lead [2-4, 13, 19, 20]. The chemical composition of the deposited layer is essentially controlled by the chemical composition of the electrolyte, while the current density and the electrolyte temperature are usually not changed.
To produce uniform solder joints with tin-lead solders, temperatures between 240 and 250 °C are required, which is well above the melting point of the eutectic alloy. These relatively high temperatures can lead to problems when producing solder joints between solar cells. For this area of application, ternary tin-lead-bismuth alloys with 2.5 to 5 % Bi are proposed in [21], which have a significantly lower melting point compared to the binary alloys. The electrolytic deposition of this alloy was carried out from methanesulfonic acid electrolytes. The chemical composition of the layer was controlled by concentrations of Sn, Pb and Bi in the electrolyte. A mixture of octylphenol ethoxylate, tamol NN8906, phenylurea, methylhydroquinone and phenolsulfonic acid were added to the electrolyte as additives.
As lead is toxic, the European Union has banned the use of lead in electronic components under the WEEE (Waste from Electrical and Electronic Equipment) and RoHS (Restriction of Certain Harzardous Substances) directives. In addition to pure tin, where problems can occur due to the formation of whiskers, binary and ternary tin alloys with silver, copper and bismuth are also described as lead-free solderable surfaces.
Deposition of tin-bismuth alloys
An alternative to lead in solder alloys is bismuth, which is completely miscible with tin over the entire concentration range. The eutectic alloy with 43 % Sn and 57 % Bi melts at 138 °C. The melting point of this alloy is therefore significantly lower than that of tin (232 °C) and the eutectic tin-lead alloy (183 °C). The addition of bismuth is an effective measure to minimize whisker formation.
The standard electrode potential for the reaction

<4>
is significantly more electropositive compared to tin (reaction <2>), so that bismuth is preferentially deposited from complex-free solutions compared to tin.
Goh and coworkers [22] carried out fundamental electrochemical studies on the deposition of binary tin-bismuth alloys on copper as the base metal from methanesulfonic acid electrolytes. Tin was added to the electrolyte as tin sulphate so that the electrolyte also contained sulphate. The investigations showed that only bismuth is deposited from additive-free electrolytes. By adding hydroquinone and gelatine, the joint deposition of tin and bismuth was possible. The chemical composition of the deposited alloy depends on the current density, whereby the bismuth concentration in the layer decreased with increasing current density. Higher current densities also led to the deposition of dendritic crystals of bismuth.
Deposition of tin-silver alloys
Tin-silver alloys are described in the literature as lead-free solderable coatings. The eutectic alloy with 96.5 % Sn and 3.5 % Ag has a melting point of 221 °C. The alloy has good strength and good creep behavior. However, the alloying ratio must be strictly adhered to, as even slightly higher silver contents lead to a significant increase in the melting temperature. Ageing can cause a thicker oxide or sulphide layer to form on the coated surface, which has a negative effect on solderability.
The standard electrode potential of the reaction

<5>
is considerably more electropositive compared to tin, so the addition of a strong complexing agent is absolutely necessary for silver. The addition of thiourea in concentrations between 20 and 200 g/L as a complexing agent is usually described in the literature [16, 23-25]. Wehner [16] investigated the deposition of tin-silver alloys on copper with an electrolyte consisting of 25 g/L tin, 1 g/L silver, 90 g/L methanesulfonic acid and various concentrations of thiourea at current densities of 50 to 1000 A/m2 in a Hull cell. As a further additive, the electrolyte contained 0.002 mol/L polyoxyethylene lauryl ether. In order for a stable silver-thiourea complex to form, an excess of thiourea to silver of at least 20 times was required. The average silver content in the layer depended on the thiourea concentration and the current density and decreased with increasing current density from approx. 50 % to 10 to 14 % Ag. The silver content in the layer was thus still significantly higher than the silver concentration in the eutectic. Point analyses carried out with EDX also revealed a highly inhomogeneous silver distribution in the layer. The tin-silver alloys deposited under these conditions therefore do not fulfill the conditions for technical use as solder alloys.
A US patent [26] describes the deposition of tin-silver alloys from methanesulfonic acid electrolytes with the addition of aromatic thiol compounds or aromatic sulfide compounds. The tin concentration in the electrolyte was 25 g/L, the silver concentration 1 or 2 g/L and the methanesulfonic acid concentration 350 or 1150 g/L. In the embodiment examples, 20 g/L of 2-aminothiophenol and 5 g/L of 2,2-dipyridyl disulphide were used as additives. The depositions were carried out in a Hull cell on polished stainless steel. The silver concentration in the deposited layers was between 3.0 and 3.6 % under optimum conditions and thus in the range of the eutectic. There is no information in the patent about the structure and chemical homogeneity of the layers produced. A further advantage mentioned in the patent is that the electrolyte remained stable for at least 60 days without the formation of precipitates.
Deposition of other metals
In studies on copper deposition from methanesulfonic acid electrolytes, Felicita et al [27] showed that the layers produced had significantly smaller grain sizes and fewer oxygen inclusions compared to deposition from sulfuric acid electrolytes. One patent [28] describes the electrodeposition of copper from alkanesulfonic acid electrolytes. Another patent [29] also describes the electrodeposition of copper in a two-stage process consisting of pretreatment and electrodeposition. According to [30], the methanesulfonic acid electrolyte is also suitable for the deposition of thin copper layers at high current densities of up to 5000 A/m2. Investigations into the use of methanesulfonic acid electrolytes in copper refining electrolysis have shown that, in contrast to conventional electrolysis from sulfuric acid electrolytes, the deposition of smooth, high-purity copper cathodes from additive-free electrolytes is possible [8].
In electroplating, cyanide electrolytes are often used for the deposition of gold and silver. Due to the high toxicity of these electrolytes and the complex wastewater treatment, a replacement by other electrolytes is desirable. In his dissertation, Ehnert [31] investigated the production of gold(I) dithiourea methanesulfonate and its use for the electrolytic and electroless deposition of thin gold layers. The electrolyte was produced by anodic dissolution of gold in an electrolyte of methanesulfonic acid and thiourea in an electrolytic cell in which the anode compartment was separated from the cathode compartment by a diaphragm. During the cathodic deposition of gold on copper, the layer turned black even at relatively low current densities. In addition, the coating adhered poorly to the substrate. In contrast, very good adhesion of solderable gold deposits could be achieved with the electroless deposition of gold using the ENIG process.
A patent [32] describes the effect of various additives on silver deposition from cyanide-free electrolytes. In most examples, a basic electrolyte with 10 to 30 g/L silver and 80 g/L methanesulfonic acid is used. The current densities were between 100 and 250 A/m2.
In his dissertation, Dressler [9] investigated the mechanism of silver deposition from methanesulfonic acid electrolytes for use in silver refining electrolysis. While only loosely adhering crystallites are deposited at the cathode in "classic" deposition from weak nitric acid electrolytes (Möbius electrolysis), compact silver deposition is possible from the methanesulfonic acid electrolyte.
Further patents describe the electrodeposition of bismuth [33] and indium and indium alloys [34] from methanesulfonic acid electrolytes with various additives.
Summary
Methanesulfonic acid is a very interesting acid for electroplating due to its high solubility for many metals, its high chemical and electrochemical stability and its low toxicity. The technical and patent literature mainly describes studies on the deposition of tin, tin alloys, copper and silver. Today, the acid is used technically in the tinning of strip steel for the production of tinplate, in tin refining electrolysis and for the production of high-purity tin-lead alloys. In recent years in particular, studies have been carried out at various research institutes in cooperation with industry on other areas of application in electroplating and for the electrochemical refining and extraction of various metals.
THE AUTHORS
Dr.-Ing. Hartmut Bombach
Institute for Non-ferrous Metallurgy and High-Purity Materials TU Bergakademie Freiberg
University Professor Dr.-Ing. Alexandros Charitos
Director Institute of Non-Ferrous Metallurgy and High-Purity Materials TU Bergakademie Freiberg
Literature
[1] US Patent 2525942 (1945)
[2] EP Patent 0172267 (1984)
[3] US Patent 4582576 (1985)
[4] US Patent 4662399 (1986)
[5] Lutropur the friendly acid BASF company publication (2011)
[6] BASF press release P115/20 from 20.01.2020
[7] Gernon, M.; Wu, M.; Buszta, T.; Janney, P.: Green chemistry (1999) 6, 127-139
[8] Bombach H.; Stelter M.; Baumbach J.; Thiere A.: A new electrolyte for copper electrorefining, Proceedings EMC, 1 (2015), 209-218
[9] Dressler, A.: Investigations on the deposition of silver from methanesulfonic acid, Dissertation TU Bergakademie Freiberg, (2020)
[10] Jordan, M.: The galvanic deposition of tin and tin alloys, Leuze Verlag, (1993)
[11] US Patent 4662999 (1987)
[12] DE Patent 69606062 (1998)
[13] US Patent 5296128 (1993)
[14] US Patent 6342148 (2002)
[15] DE Patent 3902043 (1990)
[16] Wehner, S.: Non-ionic polyethoxylated surfactants methanesulfonic acid tin and tin-silver electrolytes, dissertation TU Dresden, (2005)
[17] Martyak, N.; Seefeldt, R.: Galvanotechnik (2004), 2372-2385
[18] Rosenstein, C.: Metal Finishing, Jan. 1990, 17-21
[19] US Patent 4701244 (1985)
[20] US Patent 4885064 (1989)
[21] EP patent 3290147 (2018)
[22] Goh, Y.; Haseeb, A.; Sabri, M.: Electrochimica Acta 90 (2013), 265-273
[23] Joseph, S.; Phatak, G.: Material Science and Engineering B 168 (2010), 219-223
[24] US Patent 6099713 (2000)
[25] US Patent 2003/0024822 (2003)
[26] US Patent 5911866 (1999)
[27] Felicita, S.; Nisha, F. et al.: International Journal of ChemTech Research (2011) 3, 1318-1325
[28] US patent 6605204 (2000)
[29] US patent 7575666 (2006)
[30] US patent 6676823 (2002)
[31] Ehnert, R.: Synthesis of gold(1)-dithiourea methanesulfonate and its possible applications, dissertation TU Chemnitz, (2020)
[32] US Patent 2007/0284258
[33] EU patent 3150743 (2017)
[34] EU patent 3199666A1 (2018)


