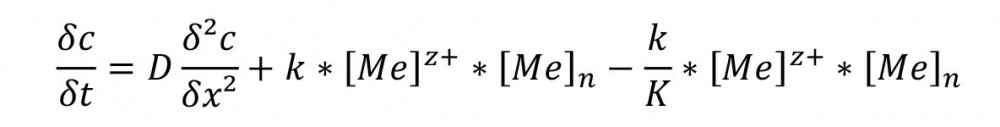Part 1: Basic approach
The development of new electrolytes for the deposition of functional layers and layer systems has so far been conventionally based on trial and error, despite a greatly increased understanding of the complex interrelationships [1]. This time-consuming and resource-intensive procedure should be faster and more targeted in times of digital penetration of all areas of technology.
Introduction
By defining suitable parameters for the overall process, it is possible to determine correlations between the electrochemistry of the deposition and the functional properties of the coatings.
The aim here should not be to find just any usable solution, but the optimum solution in the entire parameter space. As part of the research project "REACh-compliant corrosion protection through pulse plating" - ReKoPP [2] for the deposition of ternary zinc alloys, a new approach was developed for this purpose. The starting point of the project was the situation that nickel-rich zinc-nickel alloys successfully deposited by electroplating for corrosion protection in particular could be subject to application restrictions in the future as a result of the REACh regulation. corrosion-resistant ternary zinc alloys are being sought to solve the problem, whereby according to existing studies [3] alloying elements in the lower percentage range stabilize the passive layer formed by atmospheric corrosion of zinc and thus significantly improve corrosion protection. The developed and universally applicable procedure for electrolyte development is structured as follows:
- Definition of a basic electrolyte and its parameter space/working window based on thermodynamic and kinetic considerations/data.
- Conception of a robot-supported test execution with statistical test planning (DoE).
- Evaluation of the test results using neural networks and maps.
- Testing/verification of the results in the technical center and in field tests.
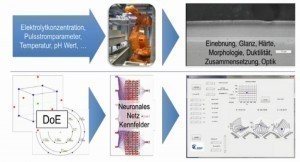
The basic relationships to point 1 are illustrated once again in Figure 1. The procedure is described in detail below:
Thermodynamics
Since electrochemical metal deposition is achieved by reducing the free metal ions and the state of equilibrium between free and complexed metal ions can be adjusted using selected complex compounds, the deposition potentials of different metal ions can be approximated - a prerequisite for the deposition of alloys. The equilibrium state between free and complexed metal ions is described by the thermodynamic law of mass action. A suitable pH working range can be derived from the protonation behavior of the complexing agent. In addition, the solubility of poorly soluble hydroxides, for example, is a prerequisite for electrolyte stability. The mathematical solution of the complex equilibrium reactions is carried out via a non-linear system of equations using algorithms developed for this purpose [4-7].
The thermodynamic equilibrium constants are determined experimentally by means of potentiometric titration. The method can be used universally for aqueous electrolytes and provides information on the different species and equilibrium constants as a function of the pH values. After calculating the free metal ion concentrations, the deposition potentials can also be determined using the Nernst equation.
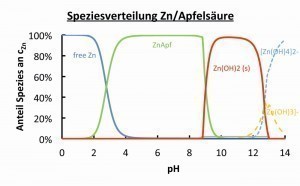
In the ReKoPP project, various amino acids, carboxylic acids and known complexing agents such as pyrophosphate, HEMPA and DTPA were investigated with regard to the distribution of zinc species and the species of possible alloying elements. As an example, the species distribution of the zinc-copper-malic acid system is shown in Figure 2. The complexing agent malic acid acts in the pH value range 6 to 8 and prevents the precipitation of zinc or copper hydroxides there, thus enabling ZnCu alloy deposition in this pH range.
Kinetics
In addition to the orienting kinetic considerations, kinetic processes such as diffusion and complex decomposition are decisive for the development of new electrolytes. To take these highly complex processes into account, a simplified model of the kinetic process of electrochemical deposition was considered.
The basis for this model is the determination of limiting current densities from the calculation of the free metal ion current through the non-moving boundary layer using Fick's first and second laws. Furthermore, the physical laws of diffusion (Fick's laws) and the chemical reaction kinetics were coupled.
When using brightener systems, the possible influences on the kinetics, for example during nucleation and microstructural layer growth, must also be taken into account. Suitable brighteners were preselected in the project by means of impedance measurements [9].
Equation 1: Reaction/diffusion equation as the basis for calculating the limiting current density according to the model in Figure 3.
Using these model concepts, it was possible to calculate the limiting current density for complex compounds with initially one metal ion and one ligand. The behavior of the limiting current density is a sufficient criterion for the kinetic design of an electrolyte system.
The model was validated by means of experimental depositions. The thermodynamic data of the complexing agents were used to calculate the optimum pH value and the necessary excess of complexing agents to produce a stable basic electrolyte.
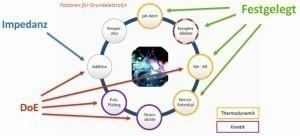
An overview of which factors of the basic electrolyte are determined by thermodynamic/kinetic considerations/data and which by measurements (impedance and statistical design of experiments - DoE) is shown once again in Figure 4.
Design of a robot-assisted test procedure (LaboRob) with statistical design of experiments (DoE)
 Fig. 5: Complexity of the electroplating processTheparameter space (electrolyte composition, pH value, potential regime and current density ranges, pulse plating value range) was defined based on the results of the thermodynamic/kinetic considerations/calculations. A large number of experiments are required to correlate these different values with the properties of the deposited layers. A statistical Design of Experiments (DoE) can be used to reduce this number. This method makes it possible to reveal complex interactions between deposition parameters and coating properties and thus to achieve optimum combinations of properties. Figure 5 schematically shows the complex dependencies in electrodeposition.
Fig. 5: Complexity of the electroplating processTheparameter space (electrolyte composition, pH value, potential regime and current density ranges, pulse plating value range) was defined based on the results of the thermodynamic/kinetic considerations/calculations. A large number of experiments are required to correlate these different values with the properties of the deposited layers. A statistical Design of Experiments (DoE) can be used to reduce this number. This method makes it possible to reveal complex interactions between deposition parameters and coating properties and thus to achieve optimum combinations of properties. Figure 5 schematically shows the complex dependencies in electrodeposition.
With this type of experiment planning and execution, all parameters are changed for each experiment, in contrast to full-factorial test series. Although this results in increased effort when calculating optimally distributed test points in the test room, the number of required tests is significantly reduced. A factor of 10 or more is achievable.
Compared to the full-factorial/gradient-based approach (only one parameter is changed to determine a local optimum), the risk of getting lost in a local optimum is reduced. Only when all statistically planned tests have been carried out is the evaluation carried out from a holistic perspective. In this way, parameter combinations that are not expected or classified as improbable can also be found, for example to form optimum layer properties.
 Fig. 6: Left: Operator-side access to the laboratory robot, bath tank, right: robot in the coating laboratory, safe working areaIn order toutilize the potential of statistical experimental design in the research of novel electrolytes, a robot-assisted experimental system was developed in the ReKoPP project as part of the LaboRob sub-project (design, operation and testing of a laboratory robot system for the research/development of electrolytes) (Fig. 6). This laboratory robot system can be used to automate essential work in the execution of primarily statistically planned experiments. The workload of the laboratory staff is considerably reduced and individual influences of the experimenters due to confusion or incomplete assignment of the experiment results are avoided.
Fig. 6: Left: Operator-side access to the laboratory robot, bath tank, right: robot in the coating laboratory, safe working areaIn order toutilize the potential of statistical experimental design in the research of novel electrolytes, a robot-assisted experimental system was developed in the ReKoPP project as part of the LaboRob sub-project (design, operation and testing of a laboratory robot system for the research/development of electrolytes) (Fig. 6). This laboratory robot system can be used to automate essential work in the execution of primarily statistically planned experiments. The workload of the laboratory staff is considerably reduced and individual influences of the experimenters due to confusion or incomplete assignment of the experiment results are avoided.
The laboratory robot contains all the elements of an electroplating system, such as bath containers for pre-treatment, coating and post-treatment, bath movement, instrumentation with sensors for checking and maintaining the fill level, pH value and concentration of species in the baths. Automatic dosing pumps ensure defined electrolyte compositions. The robot is equipped with a multi-gripper system so that it can carry out several experiments in parallel in an optimized time regime in multitasking mode. A camera for photographically recording the coated workpieces is part of the system. Using a pipetting device, this can also be used to measure the electrolyte turbidity. The current required for coating is supplied by pulse current sources.
Evaluation of the test results using neural networks and characteristic maps
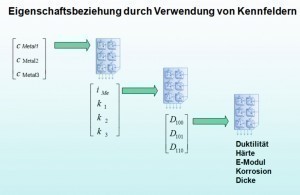
There are mathematical models for the individual phases of electroplating metal deposition, nucleation, crystal growth and the formation of a microstructure [1]. It is more difficult to calculate the physical-chemical layer properties from the material-structure relationship (structure/property relationships). Due to the complexity and the different scaling, there will probably be no holistic models in the near future to quantitatively capture the relationships shown in Figure 5. An approach with phenomenological modeling was developed in the ANSIM project (application-oriented simulation for the planning and production of customized, electrolytically produced surfaces [10]).
In order to successfully form phenomenological models, it is important to capture the characteristics of a coating for all conceivable combinations of influencing variables in the form of suitable parameters. The DoE statistical design of experiments fulfills this requirement in a special way.
The characteristic map method [11, 12] developed in the ANSIM project makes it possible to derive correlations from this initially chaotic collection. This requires an interpolation method with which a closed representation is obtained from individual interpolation points (assignment: coating parameters/coating properties). Neural networks are suitable for this in some cases high-dimensional interpolation process. A "supervised learning process" is used to assign the process parameters to the coating properties (Fig. 7).
 Fig. 7: Neural network as a phenomenological model
Fig. 7: Neural network as a phenomenological model
By interrogating the neural network, values between the support points can be retrieved. The path to correlation from the initial data in the electrolyte to the functional layer properties takes place in stages as shown schematically in Figure 8.
The training of a neural network also fulfills the properties of a "digital twin" to some extent. The digital twin stores the image of reality.
With reference to the correlations shown in Figure 8, the graphical representation of the relationships between the deposition data in the pulse regime and the textures (D) of the deposited Zn layers is shown in Figure 9.
In order to specifically determine suitable combinations of process parameters for certain layer properties, an inverse model (swapping the input and output values, Fig. 10) would have to be trained. However, this is only possible if the assignment of layer properties to process parameters is unique, i.e. if there are no multiple solutions. However, this is generally the case.
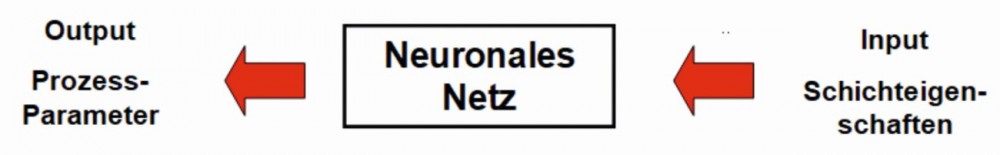 Fig. 10: Inverse query of a neural network
Fig. 10: Inverse query of a neural network
The inverse query method is not suitable for this case of ambiguous assignment, namely that the same value of a process parameter in combination with other process parameters leads to the formation of different layer properties in the entire, phenomenologically recorded observation space. For this purpose, a method was implemented with which characteristic maps can be viewed section by section in limited value ranges. Parts of the map are then examined in a restricted environment to determine "local" relationships between process parameters and layer properties. By specifying value ranges that must be part of an optimum coating result, possible value ranges of process parameters are determined, if available, compliance with which would lead to specified coating properties.
In order to illustrate the experimental path taken towards targeted electrolyte development once again, Figure 11 clearly shows the overall connection between the statistical design of experiments (DoE) and the execution of experiments with a robot-supported test facility as well as the creation of characteristic maps for the relationship between electrolyte composition/coating properties using neural networks.
Testing/verification of the results in the pilot plant
In the procedure described at the beginning, ternary alloys of the Zn-Fe-X system - with X = tin, molybdenum, manganese, copper or indium - were calculated, deposited and tested. Of the ternary systems tested, the zinc-iron-molybdenum system showed the best electrochemical characteristics, in particular for corrosion protection against steel as the base material and the highest stability. Optimal results were obtained from an alkaline electrolyte with an average current density of 1 A/dm2 and a pulse regime with ton = 50 ms and toff = 50 ms. The composition of the Zn-Fe-Mo layers showed wt.% for Fe of about 3 and Mo of about 0.5. For upscaling, Zn-Fe-Mo coatings with these parameters and with the desired functional layer properties were successfully deposited on barrel and rack components at the Gazima and B+T Oberflächentechnik technical centers [13].
The experimental data are presented in part 2 of the paper.
Note: A video about the operation of the LaboRob is available on the Klero website under "Research and development".
Acknowledgments
The authors would like to thank the German Federal Ministry of Research and Education (BMBF) and the project management organization VDI Technologiezentrum GmbH for funding the project "REACh-compliant corrosion protection by pulse plating (ReKoPP)", promotional reference 13XP5031E. Funded project partners were: B+T Oberflächentechnik GmbH, Wetzlar; Coventya GmbH, Gütersloh; Gazima GmbH, Grünhain-Beierfeld; KleRo GmbH Roboterautomation, Berlin; plating electronic GmbH, Sexau; Chemnitz University of Technology. -to be continued-


![Abb. 3: Vereinfachtes kinetisches Modell des Komplexzerfalls; [M] – Metallion | [L] – Ligand /Komplexbildner | D – Diffusions- koeffizient | K – Gleichgewichtskonstante | krück – Komplexzerfalls- konstante | δ – Diffusionsgrenzschichtdicke Abb. 3: Vereinfachtes kinetisches Modell des Komplexzerfalls; [M] – Metallion | [L] – Ligand /Komplexbildner | D – Diffusions- koeffizient | K – Gleichgewichtskonstante | krück – Komplexzerfalls- konstante | δ – Diffusionsgrenzschichtdicke](/images/stories/Abo-2020-11/thumbnails/thumbnails/thumb_gt-2020-11-0054.jpg)