Environmentally friendly coatings made of aluminum alloys are becoming increasingly interesting compared to coatings made of questionable metals such as cadmium. However, they can only be deposited using galvanic processes from aprotic electrolytes such as ionic liquids. Multianodes can be used to achieve high process stability.
Aluminum is considered a promising replacement for environmentally harmful corrosion protection layers made of cadmium. However, due to the formation of an insulating oxide layer, aluminum cannot provide continuous cathodic corrosion protection under ambient conditions and must therefore be alloyed. Aluminum-zinc alloys deposited from AlCl3/1-ethyl-3-methylimidazolium chloride offer cathodic corrosion protection under atmospheric conditions [1]. The cementation of zinc on aluminum anodes leads to a rapid decrease in the zinc ion concentration in the electrolyte and complexing agents for ionic liquids have not yet been sufficiently investigated. Therefore, an adapted process control is necessary to ensure the deposition of alloy layers with a reproducible composition.
Multianodes made of the alloying metals aluminium and zinc were used for the deposition of aluminium-zinc alloys, whereby the zinc ion content in the electrolyte could be stabilized over a long period of time. The adjustment of the current ratio between aluminum and zinc anodes also allows the targeted deposition of alloys with different compositions. Numerical simulations show that segmented multi-anodes have no influence on the cathodic current density distribution and therefore do not impair the qualitative layer growth.
Electroplating of aluminum
Electrochemical deposition of aluminum and its alloys holds enormous potential for various industrial applications. Due to their low density, high thermal and electrical conductivity, good corrosion resistance and decorative properties, these materials are widely used today.
Due to political regulation of environmentally harmful materials, e.g. cadmium (REACh regulation), interest in harmless alternatives is growing. Research into new technologies is correspondingly intensive. Aluminum is one of the most promising substitutes for cadmium in the cathodic corrosion protection of steel [2-4]. It offers excellent passive corrosion protection due to its effective self-passivation. However, cathodic corrosion protection with pure aluminum is not possible [5, 6]. The passive layer that forms in contact with air, consisting of a dense, electrically non-conductive Al2O3 layer (approx. 10 nm), interrupts the local electrical circuit and thus prevents sacrificial anodic protection. Standard methods, such as immersion tests and the neutral salt spray test, cannot reliably reflect the performance of aluminum for cathodic corrosion protection. High chloride concentrations lead to the oxide layer being disrupted and the material being activated. This results in apparently good cathodic protection, which is not the case under comparatively mild environmental conditions.
The formation of the oxide layer can be hindered by alloying. However, this leads to higher inherent corrosion of the alloys [6]. Potentially suitable alloying elements are zinc [1, 5-9] and tin [1, 6]. Aluminum-zinc alloys (AlZn) [1, 10-13] have already been successfully deposited from ionic liquids (ILs) and molten salts. The deposition of aluminum-tin alloys (AlSn) from ILs [1], mixtures of ethylene glycol/choline chloride [14], organic solvents [15] and molten salts [16, 17] has also been reported.
Aluminum has a very negative Nernst potential (-1.66 V vs. normal hydrogen electrode [18]) and therefore cannot be deposited from aqueous solutions. Non-aqueous electrolytes offer broader electrochemical windows (> 3 V) that enable the electrochemical deposition of reactive metals, e.g. aluminum, niobium and tantalum. The first electrochemical processes for aluminum deposition on an industrial scale were the SIGAL process [19-21] and the REAL process [22, 23]. Despite the successful upscaling, these processes are expensive and have high technical requirements as well as a high maintenance effort, as they are based on volatile and highly flammable compounds (toluene, ether). In addition, they have limited potential as electrolytes for the deposition of alloys, as sensitive chemical reactions are disturbed by the addition of other alloying elements [22, 23].
The deposition of metals and alloys from ILs has been intensively studied over the last decades. ILs have proven to be promising electrolytes for the deposition of aluminum and its alloys [24]. Low vapor pressure, good electrical conductivity, high solubility for many metal salts and low flammability are only some of their advantageous properties [25-27].
The electrochemical deposition of aluminum from imidazolium-based ILs, e.g. mixtures of AlCl3 and 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl), is only possible from Lewis acid electrolytes in which heptachloroaluminations (Al2Cl7-) are formed by a molar excess of AlCl3 (Eq. 1) [28-30].
4 Al2Cl7-+ 3 e- → Al + 7 AlCl4- <1>
The addition of further salts (e.g. ZnCl2) enables the deposition of aluminum alloys. During alloy deposition, the concentration of dissolved ions of aluminum and the alloying metal decreases. The use of complexing agents would enable the reproducible deposition of alloys with a defined composition by keeping the ratio of metal ions approximately constant. However, research into complexing agents for IL-based electrolytes is still in its infancy. By using soluble aluminum anodes, a continuous process can be created that extends the service life of the electrolyte, as no harmful side reactions take place at the anode (e.g. chlorine formation). At the same time, however, the alloying element is increasingly replaced by aluminum. The frequent addition of the corresponding metal salt counteracts the depletion, but also introduces chloride ions (Cl-) into the electrolyte. These react with the electrochemically active Al2Cl7-(eq. 2).
Al2Cl7- + Cl- → 2 AlCl4- <2>
This continuous conversion of Al2Cl7- to AlCl4- ultimately means that no Al2Cl7-is available for the aluminum reduction (eq. <1>) and thus brings the alloy deposition to a standstill.
Many metals can be anodically dissolved in ILs with a high current yield and thus used as soluble anodes [31]. Replacing aluminum anodes with the alloying element of the alloy to be deposited would lead to a continuous increase of the corresponding ions in the electrolyte and thus negatively influence the reproducibility of the deposition. Multianodes, on the other hand, are an elegant method of counteracting electrolyte depletion, thus stabilizing the alloy deposition while maintaining the advantages of the continuous process (avoidance of harmful side reactions) and avoiding the introduction of chloride ions into the electrolyte.
This paper discusses the use of multi-anodes for the deposition of AlZn from AlCl3/[EMIm]Cl-ILs. Single and double anodes made of aluminium or aluminium and zinc metal are compared and it is shown that segmented multi-anodes have a process-stabilizing effect but have no influence on the layer distribution.
Results of the tests
The reduction of aluminum (C1 andC2) can be seen in the cyclic voltammogram of a tungsten electrode in an AlCl3/[EMIm]Cl electrolyte (Fig. 1). When ZnCl2 is added, an additional reduction peak (C3) can be observed, which is several 100 mV more anodic than the aluminum reduction. In addition, the corresponding oxidation peaks (A1,A2 andA3) can be seen in the anodic range. The addition of ZnCl2 to the electrolyte causes the aluminum reduction and oxidation peaks to decrease. This is due to the reaction of the introduced chloride ions described in equation <2
![Abb. 1: Zyklisches Voltammogramm (100 mV s-1) einer Wolfram-Elektrode in AlCl3/ [EMIm] Cl (blau) sowie AlCl3 [EMIm] Cl mit 10 mmol l–1 ZnII (grün) bzw. mit 50 mmol l–1 ZnII (grün- gestrichelt). Der eingezeichnete qualitative Trend der Peakpositionen hat keine physikalische Bedeutung. Der Pfeil gibt die Scan-Richtung an [1] Abb. 1: Zyklisches Voltammogramm (100 mV s-1) einer Wolfram-Elektrode in AlCl3/ [EMIm] Cl (blau) sowie AlCl3 [EMIm] Cl mit 10 mmol l–1 ZnII (grün) bzw. mit 50 mmol l–1 ZnII (grün- gestrichelt). Der eingezeichnete qualitative Trend der Peakpositionen hat keine physikalische Bedeutung. Der Pfeil gibt die Scan-Richtung an [1]](/images/stories/Abo-2022-10/gt-2022-10-0202.jpg) Fig. 1: Cyclic voltammogram (100 mV s-1) of a tungsten electrode in AlCl3/[EMIm] Cl (blue) and AlCl3 [EMIm] Cl with 10 mmol l-1 ZnII (green) and with 50 mmol l-1 ZnII (green - dashed). The qualitative trend of the peak positions has no physical meaning. The arrow indicates the scan direction [1]
Fig. 1: Cyclic voltammogram (100 mV s-1) of a tungsten electrode in AlCl3/[EMIm] Cl (blue) and AlCl3 [EMIm] Cl with 10 mmol l-1 ZnII (green) and with 50 mmol l-1 ZnII (green - dashed). The qualitative trend of the peak positions has no physical meaning. The arrow indicates the scan direction [1]
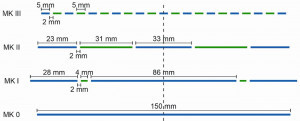 Fig. 2: Various multi-anode designs consisting of Al (blue) and Zn metal (green)Various multi-anode designs were created for the coating of steel substrates based on single anodes made of aluminum metal (Fig. 2, MK0). These differ in the area ratio of aluminum to zinc metal (Fig. 2, MKI-MKIII). At a zinc ion concentration of 10 mmol l-1 or 0.65 g l-1, AlZn layers typically have zinc contents in the single-digit weight percentage range. Based on Faraday's law (and assuming a current yield of 100 %), the proportion of charge attributable to zinc reduction during alloy deposition can be calculated. This is approx. 2 % (Fig. 3a). This resulted in the multi-anode design MKI (Fig. 2), which had a correspondingly low area fraction of zinc metal. However, it was found that the zinc ion content in the electrolyte drops so quickly that alloys with approx. 60-70 wt.% zinc would have to be deposited (secondary image in Fig. 3a), provided there are no competing reactions. This indicates that a parasitic reduction of zinc ions takes place parallel to the electrochemical reduction at the cathode. The reason for this is probably the difference in the redox potentials of aluminum and zinc of a few 100 mV (Fig. 1). This leads to the cementation of zinc on the aluminum metal of the anodes. Over time, a poorly adhering black film containing zinc forms on these. In order to compensate for the parasitic zinc reduction, the MKII multi-anode design was created with a larger surface area of zinc metal. By selectively adjusting the current ratio IAl:IZn between the aluminum and zinc metal, the zinc deposition is compensated and thus an extremely low variation of the zinc ion concentration is achieved across a large number of depositions (Fig. 3b). With increasing zinc current content, it is also easy to adjust the zinc ion concentration in the electrolyte and thus the alloy composition. This makes it possible to deposit customized alloys for different applications using a single experimental setup and without additional complexing agents. The deposition of ternary alloys is also conceivable in this way, but requires a detailed investigation of the necessary current conditions and possible cementation phenomena.
Fig. 2: Various multi-anode designs consisting of Al (blue) and Zn metal (green)Various multi-anode designs were created for the coating of steel substrates based on single anodes made of aluminum metal (Fig. 2, MK0). These differ in the area ratio of aluminum to zinc metal (Fig. 2, MKI-MKIII). At a zinc ion concentration of 10 mmol l-1 or 0.65 g l-1, AlZn layers typically have zinc contents in the single-digit weight percentage range. Based on Faraday's law (and assuming a current yield of 100 %), the proportion of charge attributable to zinc reduction during alloy deposition can be calculated. This is approx. 2 % (Fig. 3a). This resulted in the multi-anode design MKI (Fig. 2), which had a correspondingly low area fraction of zinc metal. However, it was found that the zinc ion content in the electrolyte drops so quickly that alloys with approx. 60-70 wt.% zinc would have to be deposited (secondary image in Fig. 3a), provided there are no competing reactions. This indicates that a parasitic reduction of zinc ions takes place parallel to the electrochemical reduction at the cathode. The reason for this is probably the difference in the redox potentials of aluminum and zinc of a few 100 mV (Fig. 1). This leads to the cementation of zinc on the aluminum metal of the anodes. Over time, a poorly adhering black film containing zinc forms on these. In order to compensate for the parasitic zinc reduction, the MKII multi-anode design was created with a larger surface area of zinc metal. By selectively adjusting the current ratio IAl:IZn between the aluminum and zinc metal, the zinc deposition is compensated and thus an extremely low variation of the zinc ion concentration is achieved across a large number of depositions (Fig. 3b). With increasing zinc current content, it is also easy to adjust the zinc ion concentration in the electrolyte and thus the alloy composition. This makes it possible to deposit customized alloys for different applications using a single experimental setup and without additional complexing agents. The deposition of ternary alloys is also conceivable in this way, but requires a detailed investigation of the necessary current conditions and possible cementation phenomena.
![Abb. 3: Theoretischer Stromanteil für Zinkreduktion bei Abscheidung von AlZn gegen resultierenden Zn-Legierungsanteil auf Basis des Faraday-Gesetzes und (b) Verlauf der Zinkionenkonzentration im Elektrolyten (AlCl3/ [EMIm]Cl, molares Verhältnis 1,5:1) gegen die übertragene Ladungsmenge für verschiedene Anoden und Stromverhältnisse IAl: IZn Abb. 3: Theoretischer Stromanteil für Zinkreduktion bei Abscheidung von AlZn gegen resultierenden Zn-Legierungsanteil auf Basis des Faraday-Gesetzes und (b) Verlauf der Zinkionenkonzentration im Elektrolyten (AlCl3/ [EMIm]Cl, molares Verhältnis 1,5:1) gegen die übertragene Ladungsmenge für verschiedene Anoden und Stromverhältnisse IAl: IZn](/images/stories/Abo-2022-10/gt-2022-10-0204.jpg) Fig. 3: Theoretical current ratio for zinc reduction when depositing AlZn against the resulting Zn alloy content based on Faraday's law and (b) curve of the zinc ion concentration in the electrolyte (AlCl3/[EMIm]Cl, molar ratio 1.5:1) against the amount of charge transferred for different anodes and current ratios IAl: IZn
Fig. 3: Theoretical current ratio for zinc reduction when depositing AlZn against the resulting Zn alloy content based on Faraday's law and (b) curve of the zinc ion concentration in the electrolyte (AlCl3/[EMIm]Cl, molar ratio 1.5:1) against the amount of charge transferred for different anodes and current ratios IAl: IZn
In order to ensure the greatest possible flexibility with regard to the surface areas, the segmented multi-anode MKIII (Fig. 2) was created, which consists of alternating aluminum and zinc metal strips with a respective width of 5 mm and a spacing of 2 mm. The arrangement of the strips guarantees a uniform distribution of the electric field in the electrolyte and the current density over the anode and cathode surface. The secondary current density distribution across the surface of both electrodes was calculated using numerical simulations (ComSol Multiphysics) (Fig. 4). Due to the previously discussed advantages, only the multianode MKIII is discussed below in comparison to the single anode MK0.
Compared to the edge areas, higher current densities result in the center of the anodes. On the cathode, on the other hand, high current densities are recorded primarily at the edges (Fig. 4a). This is due to the arrangement of the electrodes in relation to each other and the resulting distribution of the electric field in the electrolyte. The local current density increases linearly for both electrodes with the applied total current density, the distribution remains qualitatively identical. This means that a doubling of the total current at each location of the electrodes leads to a doubling of the local current density. As the distance between the anode and cathode increases, the current density distribution on the cathode surface does not change. However, the distribution over the anode is significantly flatter at greater distances. As a result, the dissolution of the anode is more uniform and high local current densities or potentials can be avoided. This prevents undesirable anodic side reactions (e.g. chlorine development) and increases the service life of the electrolyte.
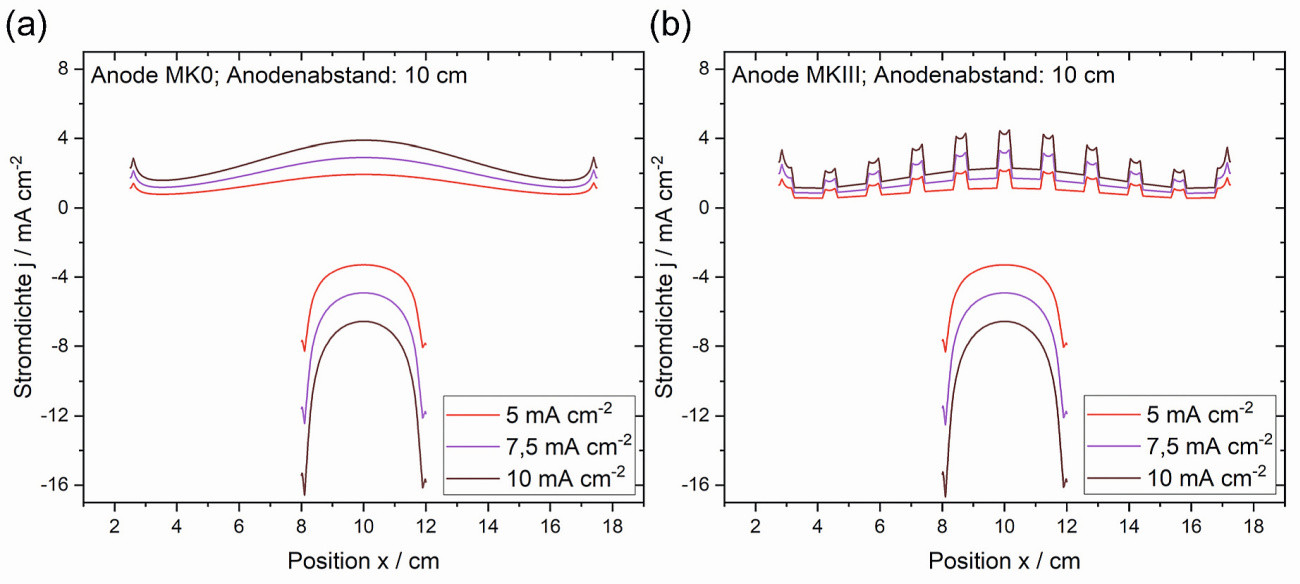 Fig. 4: Current density distribution across anode (top) and cathode (bottom) when using a (a) single anode (MK0) and (b) multi-anode (MKIII, IAI:IZn = 60:40) from simulation using ComSol Multiphysics
Fig. 4: Current density distribution across anode (top) and cathode (bottom) when using a (a) single anode (MK0) and (b) multi-anode (MKIII, IAI:IZn = 60:40) from simulation using ComSol Multiphysics
It is noteworthy that the current density distribution across the cathode surface is qualitatively the same for single and multianodes and, in particular, the distribution across the cathode surface is identical (Fig. 4a and b). Consequently, multianodes, as used here, have no influence on the current density distribution on the cathode or on the resulting layer distribution.
Summary
This paper has shown that the use of multianodes can contribute to stabilizing the electrolyte composition and increasing the reproducibility of aluminium alloy deposition in ILs. The measurement data show that cementation of zinc on aluminium metal occurs, which greatly accelerates the depletion of zinc ions, but can be compensated for by targeted zinc metal oxidation. This keeps the zinc ion concentration stable in a narrow concentration range over a long period of time. Numerical simulations show that the multi-anodes, as used here, lead to the same current density distribution on the cathode as is the case with single anodes and therefore do not affect the layer thickness distribution.
The results presented can also be transferred to the deposition of other alloys and thus open up the possibility of reproducibly depositing defined alloys from ionic liquids without additional complexing agents.
Literature
[1] Böttcher, R.: Electrochemical deposition of aluminum and aluminum alloys from ionic liquids, Dissertation, Ilmenau, 2021
[2] Legg, K.: Cadmium replacement alternatives for the Joint Strike Fighter, Rowan Technology Group, Libertyville, IL, USA, Report (2000)
[3] Metal Finishing 108 (2010) 12-20, https://doi.org/10.1016/S0026-0576(10)00012-7.
[4] Bielewski, M.: Replacing cadmium and chromium, Research and Technology Organization and NATO, RTO-AG-AVT-140 (2011) 1-22
[5] Corrosion Sci. 40 (1998) 1711-1723, https://doi.org/10.1016/S0010-938X(98)00073-0
[6] von Baeckmann, W.; Schwenk, W.; Prinz, W. (Eds.): Handbuch des kathodischen Korrosionsschutzes: Theorie und Praxis der elektrochemischen Schutzfahren, 3rd ed., VCH, Weinheim, 1989
[7] Corrosion Sci. 86 (2014) 231-238, https://doi.org/10.1016/j.corsci.2014.05.016
[8] Surf. Coat. Tech. 150 (2002) 70-75, https://doi.org/10.1016/S0257-8972(01)01508-0
[9] Corrosion Sci. 37 (1995) 79-95, https://doi.org/10.1016/0010-938X(94)00116-N
[10] Electrochim. Acta 211 (2016) 860-870, https://doi.org/10.1016/j.electacta.2016.06.081
[11] Surf. Coat. Tech. 286 (2016) 256-261, https://doi.org/10.1016/j.surfcoat.2015.10.080
[12] Electrochim. Acta 55 (2010) 2158-2162, https://doi.org/10.1016/j.electacta.2009.11.050
[13] Electrochim. Acta 46 (2000) 499-507, https://doi.org/10.1016/S0013-4686(00)00537-5
[14] Electrochim. Acta 54 (2009) 5307-5319, https://doi.org/10.1016/j.electacta.2009.04.028
[15] J. Electrochem. Soc. 134 (1987) 2425-2429, https://doi.org/10.1149/1.2100215
[16] Electrochim. Acta 100 (2013) 281-284, https://doi.org/10.1016/j.electacta.2012.09.069
[17] J. Alloys Compd. 690 (2017) 228-238, https://doi.org/10.1016/j.jallcom.2016.08.104
[18] Lide, D.R. (Ed.): CRC handbook of chemistry and physics: A ready-reference book of chemical and physical data, 76th ed., CRC press, Boca Raton, 1996
[19] Electrochim. Acta 42 (1997) 3-13
[20] Lehmkuhl, H.; Mehler, K.; Landau, U.: The Principles and Techniques of Electrolytic Aluminum Deposition and Dissolution in Organoaluminum Electrolytes, in: Gerischer, H.; Tobias, C.W. (Eds.): Advances in Electrochemical Science and Engineering, Wiley, Weinheim, 1993, 163-226
[21] Z. Anorg. Allg. Chem. 283 (1956) 414-424, https://doi.org/10.1002/zaac.19562830142
[22] J. Appl. Electrochem. 3 (1973) 321-325, https://doi.org/10.1007/BF00613040
[23] Electrochim. Acta 17 (1972) 1343-1352, https://doi.org/10.1016/0013-4686(72)80080-X
[24] Abbott, A.P.; MacFarlane, D.R.; Endres, F. (Eds.): Electrodeposition from ionic liquids, Wiley-VCH, Weinheim, 2017
[25] J. Electrochem. 21 (2015) 172
[26] J. Appl. Polym. Sci. 113 (2009) 2492-2498, https://doi.org/10.1002/app.30226
[27] Ind. Eng. Chem. Res. 47 (2008) 6327-6332, https://doi.org/10.1021/ie800665u
[28] J. Electroanal. Chem. 248 (1988) 431-440, https://doi.org/10.1016/0022-0728(88)85103-9
[29] J. Electrochem. Soc. 133 (1986) 1389-1391, https://doi.org/10.1149/1.2108893
[30] Inorg. Chem. 21 (1982) 1263-1264, https://doi.org/10.1021/ic00133a078
[31] Nat. Sci. Mater. 25 (2015) 595-602, https://doi.org/10.1016/j.pnsc.2015.11.005


