The properties of the new silver-palladium alloy are ideal for applications in the field of electrical contact surfaces and connectors. In particular, the increased demands placed on silver coatings in the field of electromobility, such as higher hardness and improved abrasion and temperature resistance, demonstrate the need for improved coatings and coating systems. Compared to established hard gold coatings, the silver-palladium alloy can also offer significant precious metal savings potential. The galvanic coating of the contact surfaces is carried out in a silver-palladium alloy electrolyte, which is free of cyanide complexes and can be integrated into a conventional electroplating process.
Introduction
Precious metals such as gold, palladium and silver, but also other PGM metals such as rhodium and platinum, are generally used for signal and power transmission in electronics due to their "noble" properties. The good, stable electrical conductivity and high resistance of these precious metals to corrosive media ensure reliable and durable contacting in the connector. The increasing number of electrical and electronic components and devices and their internal and external connections is also increasing the demand for individual contact points. Overall, this also leads to increased cost pressure and the search for more cost-effective solutions and alternative contact materials. As a result, the automotive industry, for example, is increasingly using silver surfaces. At the same time, however, the property profiles for individual applications are also changing with increased requirements in terms of hardness, abrasion resistance and sliding properties.
Silver-palladium alloys are already being used in industrial and automotive electronics. The properties of such an alloy have been described previously [1-3]. The electrodeposition of palladium-silver (60/40 wt%) was presented in [1]. Basic physical and contact properties of silver-rich alloys were investigated in [2]. In particular, however, a process for the electroplating of connectors is novel. Therefore, the aim was to offer a silver-palladium alloy with properties that are competitive with hard gold and gold-coated palladium-nickel surfaces and improve the disadvantageous properties of silver coatings. Promising results of initial investigations in practical tests have already been described [4, 5]. The silver-palladium alloy showed great potential, especially in terms of long-term stability. The new findings from further investigations are presented here.
Electrolyte properties, working range and throughput behavior
The electrolyte for the deposition of silver-palladium coatings is cyanide-free and does not contain any hard complexing agents. This makes it easy to handle in waste water treatment. All components comply with European and global chemical regulations. The properties of the electrolyte have already been described previously [5, 8, 9].
The electrolyte is designed for coil coating in continuous systems. The most important prerequisite for this is a high deposition rate due to the short exposure times. Due to an increased working temperature, strong circulation and high metal concentrations of up to 20 g/l silver or up to 12 g/l palladium, speeds of up to 5 µm/min can be achieved with the silver-palladium electrolyte.
Furthermore, the electrolyte offers a wide working window for constant coating quality and has proven to be reliable under production conditions [9]. This also includes the uniform coating composition across the entire applicable current density working range (Fig. 1). The silver content in the coating is in the range of 88-94 % at current densities of up to 10 A/dm². The palladium content is constant and is between 4 % and 5 %. Furthermore, the deposited coating contains small amounts (approx. 3 %) of a metallic brightener. The deposition rate increases linearly with the current density if there is sufficient electrolyte movement.
A stress test of the electrolyte over 2 Metal-Turn-Over (MTO) shows a stable behavior: both the deposition rate and the deposition velocity [8, 9] remain constant within a narrow range. If we look at the alloy composition in the preferred current density range, we also find a very consistent picture over 2 MTO (Fig. 2).
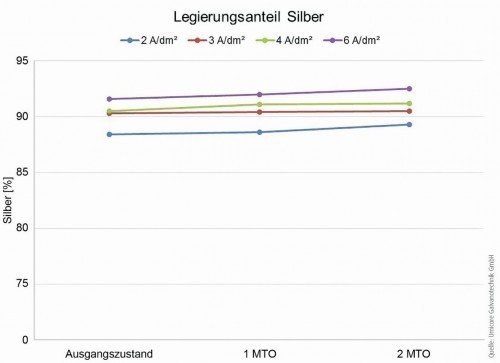 Fig. 2: Silver content in the alloy over 2 MTO as a function of current density
Fig. 2: Silver content in the alloy over 2 MTO as a function of current density
The initial experiences from system tests at customers have already been described earlier and confirmed the suitability of the electrolyte on a production scale [9]. Further tests were carried out to determine process limits in detail and to determine working parameters and, as a result, qualifications were also carried out on production systems. These tests showed that the high requirements for a production-capable process could be met. In addition to sufficient performance data for the electrolyte in terms of deposition speed and stability at elevated temperatures (up to 65 °C), it was also shown that a stable coating composition and consistent coating quality can be achieved regardless of system-related parameters such as throughput speed and flow conditions.
Coating properties Tribological and electrical properties
The abrasion resistance has already been described in earlier publications [4, 5, 8, 9]. The coefficient of friction after 500 cycles at a contact force of 50 mN with a hard gold ball as a counterpart is in the range below 0.25 and is therefore comparable to that of hard gold after the same load. Under these conditions, pure silver coatings already exhibit a significant increase in the coefficient of friction of up to 1.2 after approx. 150 cycles. The silver-palladium layer remains very stable even after heat treatment with uniformly low coefficients of friction [9], while hard gold layers exhibit similar behavior.
The contact resistance values of silver-palladium coatings after 1000 h at 180 °C are in the range of those of pure silver coatings and are well below 5 mOhm at 50 cN. No degradation of the silver-palladium coatings could be observed after temperature aging, neither in the measurement of the contact resistances nor in the friction and wear behavior [9].
Hardness
The hardness of the deposited silver-palladium layers was determined using a nanoindenter (UNAT, Asmec/Zwick). The values were determined in the initial state (as plated) and after heat aging at 200 °C for up to 250 hours. To validate the results, the palladium content in the coating was varied between 4 % and 10 %. The hardness values are in the range between 240 and 280 HV and show a fluctuation range of 5-10 %. There is no discernible trend after temperature aging, so the initial values remain the same regardless of the alloy range under consideration (Fig. 3).
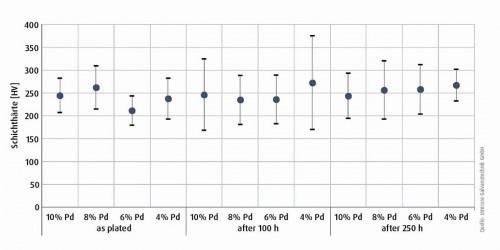 Fig. 3: Coating hardness as a function of the palladium content in the initial state and after heat treatment at 200 °C
Fig. 3: Coating hardness as a function of the palladium content in the initial state and after heat treatment at 200 °C
Grain structure and morphology
The scanning electron microscope (SEM) investigations were carried out with a Zeiss Gemini 3000 at the Research Institute for Precious Metals and Metal Chemistry (fem) in Schwäbisch Gmünd. The focused ion beam (FIB) technique was used to visualize the layer structure.
Figure 4 clearly shows the fine crystalline grain structure of the silver-palladium layer in the as-deposited state. Figure 5 shows the unchanged microstructure and morphology after heat aging at 150 °C for 1000 hours. The silver-palladium layer shows no recrystallization or interdiffusion effects and thus proves to be very temperature and long-term stable.
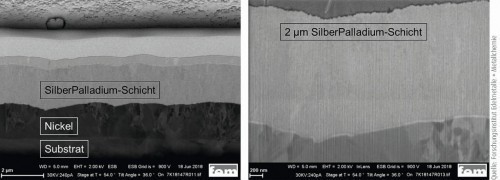 Fig. 4: FIB sections of the layer structure (substrate/nickel/flash gold/silver-palladium) (left) with enlarged section of the silver-palladium layer in its initial state (right)
Fig. 4: FIB sections of the layer structure (substrate/nickel/flash gold/silver-palladium) (left) with enlarged section of the silver-palladium layer in its initial state (right)
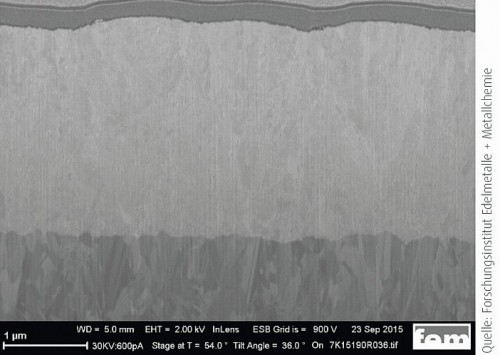 Fig. 5: FIB sections of the silver-palladium layer after heat aging at 150 °C for 1000 hours
Fig. 5: FIB sections of the silver-palladium layer after heat aging at 150 °C for 1000 hours
The investigations using EBSD (electron backscatter diffraction) were carried out by the company Matworks GmbH in Aalen. EBSD is a crystallographic technique for the structural analysis of crystals. The EBSD measuring systems are primarily used in scanning electron microscopes (SEM) and transmission electron microscopes (TEM). In the case of the investigations shown here, the EBSD measurements were carried out on FIB sections of coated samples. The FIB cross-section preparation and the orientation analysis were carried out with a Zeiss Crossbeam 540.
Figures 6 and 7 show an overview of the FIB section of the silver-palladium layer (left). This shows the layer structure substrate/nickel/flash gold/silver-palladium. Figure 6 shows the as-deposited state. The orientation of the crystals can be described as rod-shaped. The elongated grains have a width of < 1 µm. Figure 7 shows the state after heat aging at 185 °C for a period of 30 min. Here it can be seen that only a slight change in orientation and grain size can be observed. There is therefore no evidence of recrystallization of the silver-palladium layer.
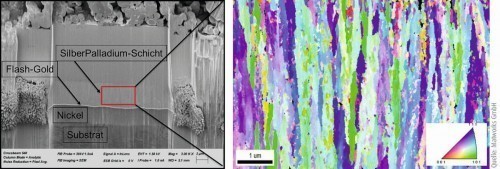 Fig. 6: Overview FIB section of the silver-palladium layer for EBSD measurement before heat aging (left) and EBSD overview, IPF, 15,000 x (right)
Fig. 6: Overview FIB section of the silver-palladium layer for EBSD measurement before heat aging (left) and EBSD overview, IPF, 15,000 x (right)
 Fig. 7: Overview FIB section of the silver-palladium layer for EBSD measurement after heat aging (left) and EBSD overview, IPF, 15,000 x (right)
Fig. 7: Overview FIB section of the silver-palladium layer for EBSD measurement after heat aging (left) and EBSD overview, IPF, 15,000 x (right)
To further investigate the layer properties, the silver-palladium coatings were examined using SEM-EDX on FIB sections. These were carried out at the Research Institute for Precious Metals and Metal Chemistry (fem) in Schwäbisch Gmünd. A Zeiss Gemini 3000 with EDX detector was used for this purpose. By combining the imaging scanning method (SEM) with energy dispersive X-ray analysis (EDX), it is also possible to determine the distribution of the various elements on a predefined surface. Figure 8 shows the layer structure in the SEM in the left area: A flash gold layer (Au-flash) and finally the silver-palladium layer can be seen on the nickel sulphamate layer (NiS). The distribution patterns of the various elements are shown on the right-hand side of Figure 8. It can be seen that the elements silver, palladium and the metallic luster additive are distributed very homogeneously over the area under consideration.
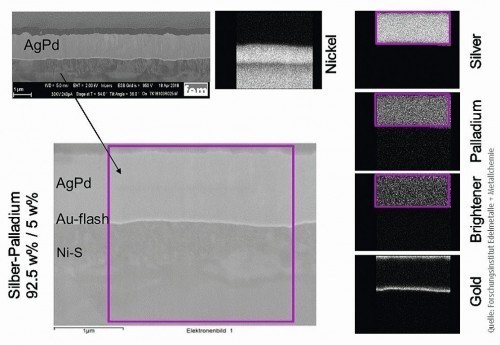 Fig. 8: Element mapping in the scanning electron microscope (SEM-EDX)
Fig. 8: Element mapping in the scanning electron microscope (SEM-EDX)
Crystal structure analysis
The X-ray diffraction (XRD) investigations were carried out at the Research Institute for Precious Metals and Metal Chemistry (fem) in Schwäbisch Gmünd. The XRD technique is used to analyze the crystal structure of silver-palladium coatings. An X-ray diffractometer from Bruker AXS (model D8 Discover) in GADDS configuration ("General Area Detector Diffraction System") was used for this purpose. In crystallography, the Scherrer equation is used to establish a connection between the width of the peak in the diffractogram of a solid and the size of the crystallites. Typically, layer thicknesses of approx. 5 µm were chosen to avoid the overlapping of reflections from the base material with those of the silver-palladium layer. The layer sequence of the samples examined here was 2 µm silver-palladium directly on brass.
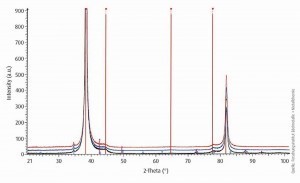 Fig. 9: X-ray diffractogram of the silver-palladium layer as deposited and after heat aging at 180 °C after 500 and 100 hoursAs Figure 9 shows, only reflections of the silver can be observed. The lattice parameters of the palladium are very similar to those of the silver and cannot be distinguished. It can therefore be concluded that both palladium and the "metallic luster additive" could be embedded in the lattice of the silver. The diffractogram shows a preferred orientation along the <111> plane. Heat aging for up to 1000 hours at 180 °C does not result in any visible changes in this respect, such as new reflections of other phases or a change in the reflection width. This is a clear indication of a constant crystallite size.
Fig. 9: X-ray diffractogram of the silver-palladium layer as deposited and after heat aging at 180 °C after 500 and 100 hoursAs Figure 9 shows, only reflections of the silver can be observed. The lattice parameters of the palladium are very similar to those of the silver and cannot be distinguished. It can therefore be concluded that both palladium and the "metallic luster additive" could be embedded in the lattice of the silver. The diffractogram shows a preferred orientation along the <111> plane. Heat aging for up to 1000 hours at 180 °C does not result in any visible changes in this respect, such as new reflections of other phases or a change in the reflection width. This is a clear indication of a constant crystallite size.
In a further XRD investigation, the change in the crystal structure up to a maximum temperature of 300 °C was observed by means of in-situ heat treatment. For this purpose, the sample was examined in the as-deposited state and after heat aging at a gradually increased temperature in the X-ray diffractometer. The temperature was increased in steps of 50 °C. The measuring time and thus also the holding time was approx. 1 hour per temperature step. The heating rate was 20 °C/minute.
In order to record only the silver-palladium coating, the diffractograms were recorded in the initial state and after completion of the temperature treatment using high-resolution grazing incidence X-ray diffraction (GIXRD) on a D8 Discover Da Vinci X-ray diffractometer from Bruker AXS. The samples used for this had the layer sequence brass substrate/2 µm nickel-gold-flash/2 µm silver-palladium.
Figure 10 shows the diffractograms of a silver-palladium layer after exposure to temperatures up to 200 °C. A reduction in the lattice parameters of the silver phase from 4.07 to 4.06 Å can be observed after annealing at 200 °C. However, no changes with regard to the phases or the microstructure can be recognized. As already described, the preferred texture is the <111> orientation.
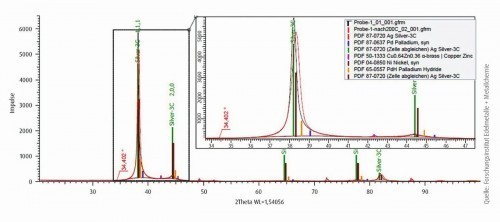 Fig. 10: Diffractogram of the silver-palladium layer after temperature treatment up to 200 °C
Fig. 10: Diffractogram of the silver-palladium layer after temperature treatment up to 200 °C
The change in the diffractogram during the gradual increase in temperature up to the maximum value of 300 °C is shown in Figure 11 . The high-resolution GIXRD technique was used to determine the crystallite size; the corresponding diffractogram is shown in Figure 12 . The instrumental reflection broadening was taken into account by measuring a LaB6 standard. The crystallite size was calculated using the Scherrer equation. The growth mainly occurs at temperatures above 200 °C. The calculation was made according to the Scherrer equation from the peak width of the corresponding reflexes. In the as-deposited state, the crystallite size is 23 nm; after reaching the maximum temperature of 300 °C, the crystallite size is 37 nm. The crystallite size thus increases only slightly.
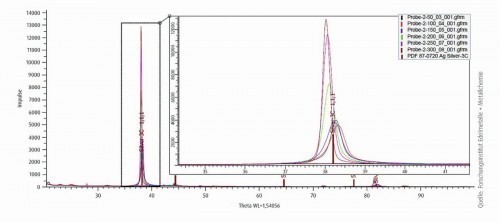 Fig. 11: Diffractogram during the gradual in-situ temperature increase (50 to 300 °C in 50 °C steps; 20 °C/min.)
Fig. 11: Diffractogram during the gradual in-situ temperature increase (50 to 300 °C in 50 °C steps; 20 °C/min.)
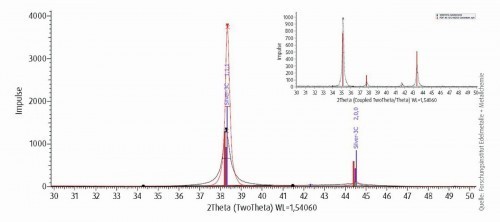 Fig. 12: High-resolution diffractogram of a silver-palladium layer to determine the crystal size in the initial state and after heat aging at 300 °C
Fig. 12: High-resolution diffractogram of a silver-palladium layer to determine the crystal size in the initial state and after heat aging at 300 °C
It can therefore be seen that after deposition, the silver-palladium layer investigated is present as a silver solid solution with a preferred orientation of the <111> crystallites towards the sample surface. During temperature treatment, the lattice parameter of the unit cell of the silver solid solution decreases and the crystallite size increases slightly. No change can be observed with regard to the phases or the microstructure.
Further development of connector contacts
Technical connectors are generally used with established contact layers such as hard gold, gold-plated palladium-nickel alloys and silver layers, but have limitations when it comes to new requirements. Although hard gold and gold-plated palladium-nickel alloys are very reliable contact layer systems, they are also expensive end surfaces.
Compared to gold, silver is a cost-effective surface and provides the best electrical conductivity. However, under end-of-life conditions, which require high abrasion resistance, diffusion and temperature stability, silver reaches its limits.
The silver-palladium alloy (brand name ARGUNA®-Alloy 1, see Fig. 13) represents an attractive alternative for optimizing plug contacts and, with the technical properties described above and the lower precious metal costs, also opens up new application possibilities.
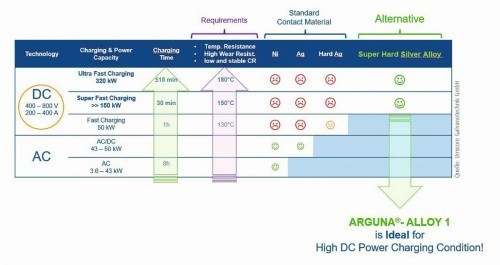 Fig. 13: Requirements profile for charging technology (EV charging)
Fig. 13: Requirements profile for charging technology (EV charging)
One possible new field of application is the electrification of vehicles. Hybrid and electric vehicles are charged electrically. Short charging times are one of the fundamental prerequisites for good market acceptance, especially for fully electric vehicles.
The requirements profile shown in Figure 13 shows the enormous charging capacities that will be required for electric refueling in the future and which contact materials are currently being used.
From the "Super Fast Charging" charging technology and upwards, contact materials are exposed to high energy loads. Tests and simulations show that temperatures of at least 150 °C are already reached at the contacts and, depending on the charging power, the high-current components are exposed to temperatures of up to 200 °C. Such high-current charging connector systems are required to withstand at least 10,000 mating cycles. The previously used contact layer system with nickel intermediate layer and silver end layer reaches its limits here. Above a temperature of 160 °C, silver shows a pronounced recrystallization of the grain structure. In addition, delamination of the silver layer can be observed due to diffusion of atmospheric oxygen and the formation of nickel oxides, for example (see Fig. 14) [9].
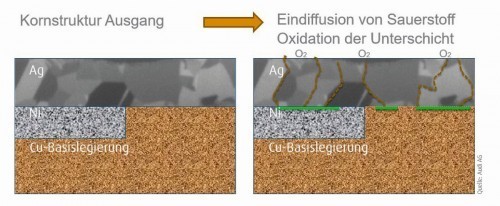 Fig. 14: Oxygen diffusion through a silver coating after temperature exposure at 160 °C
Fig. 14: Oxygen diffusion through a silver coating after temperature exposure at 160 °C
Investigations of such silver coating systems showed changes in resistance and abrasion behavior over the test duration. This resulted in premature malfunctions or complete failure during electrical charging. In the future, the high-current charging plugs should be able to withstand well over 10,000 charging cycles and have runtime reserves. Compared to a silver-plated plug, the silver-palladium system achieved over 30,000 mating cycles, showed no delamination and stable resistance behavior with sufficient runtime reserves.
Higher requirements apply to the high-current components in electric vehicles: under end-of-life conditions, mating cycles, vibration and contact transition resistance during ageing at 180 °C over 1000 hours are considered. When the full system power is called up, high-current connectors reach temperatures of up to 200 °C. Contact systems coated with silver-palladium showed an increased service life of up to 40 % in direct comparison with silver coatings. This result could also be achieved without an additional nickel intermediate layer. Nickel-free connector systems are realized with palladium intermediate layers due to their excellent properties as a diffusion barrier. However, the high precious metal costs are a disadvantage.
Summary
Compared to palladium, silver-palladium layers are a cost-effective alternative. At temperatures of up to 200 °C, the coating properties remain constant, such as unchanged grain structure, stable contact resistance and constant hardness values. Furthermore, silver-palladium acts as a diffusion-stable barrier layer and prevents oxygen diffusion, for example.
The test results described show excellent abrasion resistance and a significantly increased service life of assembled connector systems.
In summary, the special properties of the silver-palladium alloy open up new options in the design and application of contact systems, such as those required for high-current applications in the next generation of electric mobility.
Literature
[1] F. Nobel: Electroplated Palladium-Silver (60/40 wt%) Alloy as a Contact Material, IEEE Transactions on Components, Hybrids and Manufacturing Technology, V. CHMT-8, No. 1, March 1985, 163-172
[2] N. Harmsen; K. Schiff: The Basic Physical and Contact Properties of Silver-Rich Ag-Pd Alloys with Base Metal Additions, Proceedings of the HOLM Conference on Electrical Contacts, 1977, 93-97
[3] D. Ruehlicke; H. Fruend; G. Baer: Tarnish Layer Formation on AgPd Contact Surfaces, Proceedings of the 10th International Conference on Electrical Contacts, 1980, 721-741
[4] M. Myers; H. Schmidt: Connector Level Performance Evaluation of a New High Speed Reel to Reel Electroplated Silver Palladium Alloy Contact Finish, ICEC 2014, Dresden, 6-101
[5] F. Talgner: Novel Silver-Palladium Electrolyte for Electrical Cont[1] acts, ICEC 2014, Dresden, 91-95
[6] M. Myers: Overview of the use of Silver in Connector Applications, TEC 503-1016
[7] M. Myers: The Performance Implications of Silver as a Contact Finish in Traditionally Gold Finished Contact Applications, Tyco Electronics Innovation Conference, 2009
[8] F. Talgner; U. Manz; S. Berger; B. Weyhmüller; A. Pfund: New silver-palladium electrolyte for electrical contacts, Jahrbuch Oberflächentechnik, 71, 2015, 37-42, Eugen G. Leuze Verlag
[9] F. Talgner; M. Myers; H. Schmidt: Electroplated silver-palladium as a contact surface, Galvanotechnik 5/2017, 910-917, Eugen G. Leuze Verlag


