Even before the restrictions imposed by the REACh Regulation, there were efforts to replace chromium(VI) with chromium(III) applications. Although the results were not satisfactory, work on alternative solutions is still being concentrated on. Employees of the BIA Group provide information on the status of development.
Plastics are treated with chromium sulphuric acid during the electroplating process. The chromium trioxide dissolves the butadiene phase from the ABS plastic in the presence of sulphuric acid. The use of chromium trioxide has been subject to
REACh legislation, the use of chromium trioxide has been subject to authorization by the EU Commission since September 2017 [1]. However, the issue of substituting chromium(VI) compounds was not itself initiated by REACh; rather, this is a general point in the continuous optimization of processes in the coating industry.
Within the BIA Group, the first chromic acid-reduced stain "Silken Etch" was already tested under series conditions in Solingen in 2007 in cooperation with the company Coventya, as well as a chromium(III) electrolyte for chrome plating [14]. However, it was determined at the time that the significant reduction in the oxidizing agent could not be compensated for by the other ingredients of the pickling agent and the process control. The requirements for adhesion and climate change stresses according to automotive standards were not met. For this reason, a switch was made back to the tried and tested pre-treatment. However, the development of alternatives is still being pursued and measured against the requirements of the qualification tests. To this end, it is increasingly important not only to discuss the general debate on the chemical solution to the pre-treatment requirements, but also to keep an eye on the industrial implementation of the alternatives.
Various approaches under development
 Fig. 2: Examples of series components evaluated in the alternative processesSemi-preciouschromium compounds are an important component in the pre-treatment of plastics for electroplating due to their oxidation potential. The butadiene component in acrylonitrile butadiene styrene (ABS) is oxidatively dissolved from the plastic surface by the chromium sulphuric acid. The chromium is reduced from hexavalent to trivalent chromium by the oxidation of the butadiene. As a reversible redox reaction, the chromium(III) can then be oxidized back to chromium(VI) to make it available for the process again. The high oxidation potential of chromium(VI), the simple composition of the electrolyte of chromic acid and sulphuric acid and the almost unlimited service life are important features of this proven technology. In addition, the peripherals required for the safe operation of the bath and the oxidation cell are very easy to handle in production.
Fig. 2: Examples of series components evaluated in the alternative processesSemi-preciouschromium compounds are an important component in the pre-treatment of plastics for electroplating due to their oxidation potential. The butadiene component in acrylonitrile butadiene styrene (ABS) is oxidatively dissolved from the plastic surface by the chromium sulphuric acid. The chromium is reduced from hexavalent to trivalent chromium by the oxidation of the butadiene. As a reversible redox reaction, the chromium(III) can then be oxidized back to chromium(VI) to make it available for the process again. The high oxidation potential of chromium(VI), the simple composition of the electrolyte of chromic acid and sulphuric acid and the almost unlimited service life are important features of this proven technology. In addition, the peripherals required for the safe operation of the bath and the oxidation cell are very easy to handle in production.
New developments for the substitution of chromium(VI) in production must also be able to generate this oxidative effect on the surface. This ensures the adhesive bond to the metal layer and the activation of the plastic surface, and alternatives must also be able to achieve these properties. The availability of oxidizing agents within the chemical elements is limited. Known options are ozone (O3) and metallic anions such as permanganates (MnO4-) or hydrogen peroxide (H2O2). In addition to the oxidizing power to dissolve butadiene, the applicability on a large industrial scale is of course also important. Here, gaseous substances have massive disadvantages in terms of plant design and safety aspects, which is why current development approaches focus on aqueous solutions [15].
The development processes currently available often use metallic anions in combination with strong acids. In addition to the actual oxidation process, the acids also force a chemical functionalization of the surface. At the same time, the acrylonitrile-styrene matrix is slightly attacked, which increases the cleaning effect and the accessibility of the embedded butadiene. Chromium(VI) has an oxidation potential of 1.33 V. The oxidation potential of higher-value manganese compounds is strongly dependent on the pH value and, as an example, ranges from 0.564 V (pH = 14) to 1.51 V (pH = 0) for potassium permanganate. Accordingly, strongly acidic manganese solutions can reach the oxidation potential of chromium sulphuric acid. Patented manganese-based pretreatment processes use higher-value manganese compounds as oxidizing agents, whereby the oxidation level varies depending on the process [12]. In order to reduce the autocatalytic decomposition to manganese dioxide (MnO2), these are stabilized with sulphuric acid, phosphoric acid or other additives. These approaches aim to substitute chromosulfuric acid by a similar oxidation process and also to keep the corresponding plant periphery similar. [2-4]
Alternative processes
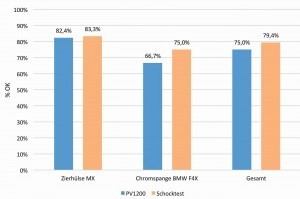 Fig. 3: Results of adhesion tests after chromium-free pre-treatment In contrast to these processes, which are based on earlier developments and PCB technology, there are alternative processes that depart from these familiar procedures. For example, there are approaches that rely on chemical-physical displacement processes similar to a foaming process in the morphological surface layer [5, 6]. This is still a strongly acidic solution, but the chemical process for pretreating the surface is not exclusively due to oxidation of the butadiene. In addition, there is hardly any attack on the SAN matrix [6]. Furthermore, there are projects and research projects that aim to apply a conductive layer by painting or an immersion process or to create a surface with an affinity for palladium by polymerization [9, 10]. In addition to electroplating ABS without chromium trioxide, the aim here is to make other polymers accessible for this coating process. While the physical displacement process relies on a strongly acidic solution on an aqueous basis and can be integrated into the current process sequence, the other two processes mentioned require a new concept for pre-treatment. This can be an opportunity for general optimization. However, the process must be tested in series production to justify investment in new plant construction. From a current perspective, these processes are still classified as research and development. [10, 11]
Fig. 3: Results of adhesion tests after chromium-free pre-treatment In contrast to these processes, which are based on earlier developments and PCB technology, there are alternative processes that depart from these familiar procedures. For example, there are approaches that rely on chemical-physical displacement processes similar to a foaming process in the morphological surface layer [5, 6]. This is still a strongly acidic solution, but the chemical process for pretreating the surface is not exclusively due to oxidation of the butadiene. In addition, there is hardly any attack on the SAN matrix [6]. Furthermore, there are projects and research projects that aim to apply a conductive layer by painting or an immersion process or to create a surface with an affinity for palladium by polymerization [9, 10]. In addition to electroplating ABS without chromium trioxide, the aim here is to make other polymers accessible for this coating process. While the physical displacement process relies on a strongly acidic solution on an aqueous basis and can be integrated into the current process sequence, the other two processes mentioned require a new concept for pre-treatment. This can be an opportunity for general optimization. However, the process must be tested in series production to justify investment in new plant construction. From a current perspective, these processes are still classified as research and development. [10, 11]
Process sequence and system integration
As most processes do not only require the substitution of chromium sulphuric acid, Table 1 shows a comparison of the process sequences according to developer specifications. As the process sequence shows, the alternative processes with manganese as an oxidizing agent are also dependent on a swelling step. This is intended to strengthen the general pickling attack by swelling the polymer chains. It should be noted that these processes take considerably longer in the time required for pre-treatment. Thus, the swelling process is added as an active bath, which also requires a rinsing process. In addition, the expected exposure time required in the manganese pickling process increases significantly compared to the chromosulfuric acid pickling process (8-12 min vs. 15-23 min).
|
CrO3-based process |
Mn-based processes |
Foaming |
Coating |
||
|
Process 0 |
Process 1 |
Process 2 |
Process 3 |
Process 4 |
Process 5 |
|
Chromosulfuric acid |
Mn(VII) in a weakly acidic environment |
Mn in various, higher oxidation states in a strongly acidic environment |
Caro's acid in a strongly acidic environment |
Grafting |
|
|
Attachment to electroplating racks |
Mounting on electroplating racks |
Mounting on electroplating racks |
Mounting on painting racks |
||
|
Chrome-sulfuric acid pickling |
Swellers |
Physical foaming |
lacquering |
||
|
|
Manganese pickling |
|
UV curing |
||
|
Neutralization |
Neutralization |
|
Transfer to electroplating racks |
||
|
|
optional: conditioner to increase Pd adsorption |
|
|
||
|
Pre-dip activation |
Pre-dip activation |
Pre-dip activation |
|
||
|
Pd activation |
Pd activation |
Pd activation |
Pd activation |
||
|
Accelerator |
Accelerator |
Accelerator |
Accelerator |
||
|
electroless nickel |
electroless nickel |
electroless nickel |
electroless nickel |
||
|
Electrolytic coating according to industrial standards (Cu/Ni/Cr) |
|||||
The process steps and duration have a critical disadvantage when retrofitting existing and building new systems. Industrial series systems with a coating capacity of approx. 40-80 m²/h are operated in cycles. This means that the process baths are operated in the appropriate sequence by an automatic machine at fixed cycle times. The components are placed on product carriers and an automatic lifting trolley follows the process sequence. To optimize the sequence, the capacity of the system and to ensure constant process conditions, the dwell times are fixed for each process bath. For example, a new carrier is moved into the system every 5 minutes and, accordingly, every other product carrier must be lifted one position further every 5 minutes with a certain tolerance. If times of 10 minutes are now required in the chromium sulphuric acid, at least two positions must be carried out as pickling. If 15 min are to be realized, three pickling positions are necessary. This means that alternative processes with a pickling time of more than 20 minutes alone require at least two additional positions in a line in order to achieve the same coating capacity as the conventional pre-treatment process. This naturally requires higher investment sums or, when converting existing systems, problems with integration due to the limited installation space available. The additional positions due to swellers and the necessary rinsing steps between the active belts must also be taken into account. When planning a system with a cycle time of 4 minutes, 6-11 additional positions are required compared to the conventional manganese process. This is a critical factor when retrofitting existing systems, as the space in the existing production halls is usually already well utilized.
In conventional pre-treatment containing chromic sulphuric acid, the chromic acid contained in the stain inhibits the PVC frame plastisol. This prevents the frame rubber coating from also being germinated in the activation step and thus being metallized in addition to the plastic components. In the case of chromium-free conditioning, this crucial component is missing, which is why an additional bath is required to inhibit the frame metallization (frame inhibitor or frame protection). Depending on the process, this treatment step is usually positioned after the demetallization of the electroplating racks or during the pre-treatment process. This adds an additional active bath and a further rinsing position to the overall setup, which makes implementation in existing systems on a conventional basis even more difficult.
Evaluation: process reliability and adhesion
In the coating of plastics, pre-treatment has the task of ensuring the adhesion mechanism between plastic and metal by activating and forming a cavern structure. To this end, palladium adsorption and nickel plating enable electrical conductivity in order to deposit thicker metal layers during the subsequent process. The current component designs, the requirements for adhesion and climate change stress as well as the different plastic substrates already pose a challenge for the conventional process. High flow orientations in injection moulding and distinctive designs already lead to difficult pre-treatment, as the butadiene can no longer be oxidized due to post-crosslinking in places or high orientation and polycarbonate contents lead to adhesion problems. Conventional process control is already facing challenges here and the substitution processes must of course also meet these requirements. Accordingly, current series components in the BIA Group were used to compare the processes. One of the most important evaluation criteria is the climate change test using the example of the PV1200 (8 cycles) in accordance with TL528 [13].
The series components were produced in various processes using chrome-free pre-treatment in the technical center. The other coatings after electroless nickel were applied in the series system in parallel to conventionally pretreated components in accordance with the coating thickness specifications. In addition to tests with single-component parts, multi-component parts were also considered. However, as the selectivity in the multi-component process and the coating of PC/ABS did not lead to satisfactory results during the tests, the adhesion tests concentrated on 1-component ABS components. In many cases, overchroming into the polycarbonate component or open areas on the components could not be avoided during the coating process, which cannot be considered a reliable process for series production (Fig. 1).
The 1K ABS components could be coated in a manganese-based alternative process. Various components, e.g. from the gear knob area as well as rotary actuators and decorative frames, were considered here. When fully coated, these were then evaluated in the shock test in accordance with DBL8465 and the PV 1200 climate change test in accordance with TL528 (Fig. 2).
The following diagram(Fig. 3) shows the results for the two components in Figure 2 after the two specified tests. The reference and evaluation criterion here is the conventional pre-treatment, in which no component showed delamination, cracks or similar defects in the tests.
Overall, an OK rate of 79.4 % was recorded in the shock test and 75 % in the PV1200 climate change test. The components showed small cracks and bubbles. These are located in areas with high material shear and in geometries with sharp edges and thin wall thicknesses. In general, however, it should be noted that the defects were significantly smaller than the components tested in benchmark tests over the last 4 years [7; 8]. Progress can therefore be seen, but series safety cannot yet be established. Figure 4 shows examples of adhesion problems.
Fig. 4: Component defects (delamination) after climatic cycling stress
Conclusion
In general, the development of alternative processes for the pre-treatment of plastics for electroplating is making progress. There are more polymer types for which full-surface coating is possible. The performance of pre-treatment has also improved slightly. However, this is still no comparison with conventional pre-treatment. The process is still clearly at the development stage, and series reliability on an industrial scale has not yet been tested. The manganese processes currently show the better performance.
The FGK's benchmark tests in recent years [8] show that other processes such as a conductive polymer layer, physical foaming of the surface or similar are still a long way from a generally saleable surface quality and adhesion. Nevertheless, these processes are very interesting as they offer other advantages. For example, they are shorter than the cost-intensive manganese processes. The disadvantages of manganese-based processes are the very high proportions of sulphuric acid and phosphoric acid, which must be taken into account in terms of occupational safety and are hygroscopic process solutions. The substitution of hexavalent chromium compounds from plastics pre-treatment is correct and sensible in view of the carcinogenic properties. However, this should not be done using processes that can only be operated with extremely high safety costs and problems with peripherals and quality. An objective evaluation of substitution processes in practice is necessary here. For this reason, BIA participates in benchmark tests within the industry and in research projects for the active development of process-safe alternatives. Investments are also being made at the Solingen site in the ability to evaluate new processes under series production conditions. New and flexible plant technology in coordination with the process developers should make it possible to evaluate alternative processes in parallel to conventional production and to develop the necessary scale-up of the processes. In this way, the future viability of plastic electroplating is being actively shaped and developed.
Literature
[2] Schütte, A.: Constructive adhesive bond in chromium(VI)-free plastic pretreatment, ZVO Oberflächentage, Berlin, 2019
[3] Lemke, D.: On the way to chromium(VI)-free pretreatment, Journal für Oberflächentechnik, 2014
[4] Voß, T.: Chromium(VI)-free pretreatment of plastics for decorative metallization, ZVO Oberflächentage, Leipzig, 2018
[5] Hofinger, J.: Beyond ABS - Process-compatible chemical pretreatment of special plastics, ZVO Surface Days, Leipzig, 2018
[6] Hofinger, J.: Foaming instead of etching - status of the development of chromium(VI)- and manganese-free plastic pretreatment, Womag 11, 2018
[7] Heinzler, F.A.: Status of chromium-free plastics pretreatment, Chrom 2020, Hofheim, 2017
[8] Heinzler, F.A.; Klaiss, C.: Conditioning with Cr(VI)-free alternatives, Chrom2030, Stuttgart, 2018
[9] Lehmann, D.; Nagel, J.; Zimmermann, P.; Schlenstedt, K.: Chromium(VI)-free process for plastic electroplating of ABS surfaces, Galvanotechnik 1, 2018
[10] Och, R.; Scheuermann, K.; Meinhardt, J.; Rose, K.; Final report on palladium substitution by direct polymer electroplating, (PaSuP), (AZ 29737)
[11] Approved funding application Project: Directly electroplatable polymer (digaP) Funded by the Federal Ministry of Education and Research (BMBF) in the funding program KMU-innovativ: Materials research (ProMat_KMU)
[12] Noffke, F.; Werner, C.; Königshofen, A.: US201716461110, Chromium Free Plating on Plastic Etch, 27.02.2017
[13] N.N.: Plastic parts, chrome-plated: Material requirements No. TL 528, Volkswagen AG, Wolfsburg, 2015
[14] Coventya, DE000060205258T2, Pretreatment of plastic materials, 27.07.2005
[15] Dr. Henne, S.; Gas phase conditioning of plastics, Plastics electroplating symposium, Lüdenscheid, 2018






