Question: We have had a new system for phosphating steel parts for around two years. In three-shift operation, parts are almost exclusively coated with a zinc phosphate layer for a customer and usually oiled with an emulsion. So far there have been no complaints, but now the customer would like a measurement report stating the local coating thicknesses. As far as we know, this is not possible. Can you recommend any relevant standards or technical literature that can prove this?
Answer: It is also possible to measure the coating thickness on phosphated surfaces, but this is unusual. In order to understand the measurement methods, you first need to know the coating structure and the coating properties.
The phosphate coating
Phosphate coatings are so-called conversion coatings. These layers are created chemically by dissolving part of the surface of the substrate. The phosphate anion of this layer is always supplied from the solution, the cation from the solution and/or from the carrier material. A thin, crystalline salt layer of metal phosphates forms on the surface of the workpieces.
In the past, phosphating processes were divided into layer-forming phosphating processes and non-layer-forming phosphating processes, depending on whether or not all components of the layer produced originate from the treatment solution.
The layer-forming phosphating processes include zinc, calcium and manganese phosphating. Non-layer-forming phosphating processes included alkali phosphating and iron/steel phosphating. However, a layer is also created in the non-layer-forming processes. For this reason, a distinction is now made between conversion layer-forming processes and layer-depositing processes.
In the layer-depositing phosphating processes, the cations and anions of the generated layer are ingredients of the treatment solution and are deposited as a compound on the surface. This occurs in zinc, calcium and manganese phosphating.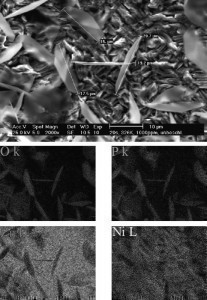 The individual crystals of the phosphate layer are clearly visible under the microscope. They stand out from the surface and are recorded when measuring the roughness
The individual crystals of the phosphate layer are clearly visible under the microscope. They stand out from the surface and are recorded when measuring the roughness
In the conversion layer-forming phosphating processes, only the anions are ingredients of the treatment solution. The cations are formed when the base material is dissolved. Compounds between these cations and the anions of the treatment solution are deposited on the surface.
Conversion layers are formed in a rearrangement process in which the metal ions are detached from the surface to be coated and deposited again in conjunction with the anions from the reaction solution. This takes place during alkali phosphating and iron/steel phosphating.
The salt layer is firmly bonded to the base metal and contains numerous capillaries and cavities, which give it good absorption capacity for fats, oils and paints. This salt layer usually consists of secondary and tertiary iron, zinc, calcium or manganese phosphates or mixtures of these salts and oxides.
The phosphating process produces coarsely crystalline layers on the surface, which are never completely dense due to their texture. Therefore, phosphating alone does not guarantee sufficient corrosion protection. The pores must always be sealed by special post-treatment.
The result of layer-depositing phosphating is already influenced by the previous history of the materials to be coated. Impurities in the melt or in corresponding rolling processes can create local areas of differing attackability. This also applies to hardening processes. This results in inhomogeneous phosphate layers. A further risk is posed by anti-corrosion greasing for storage and transportation processes. These greases must be removed by suitable process steps before phosphating.
However, most faults can occur during the actual coating process. Here they are influenced by the system configuration and the control of the process solutions. The structure of the coatings can be improved, for example, by targeted external influences during phosphating. Vibration or ultrasonic influences can be used to achieve finer-grained structures.
The zinc or manganese phosphate layers consist of 95 to 98 % secondary or tertiary zinc or manganese phosphates and 2 to 5 % iron phosphates. Depending on the type of materials and the surface properties of the workpieces produced from them, as well as their mechanical and chemical treatment prior to phosphating, the composition of the phosphating bath and the working conditions during phosphating, layers of different mass per unit area and/or different apparent density are produced.
Layer thickness / layer weight
Due to the layer properties, which are more similar to a sponge than a classic galvanic layer, it is difficult, but not impossible, to determine the local layer thickness. As a rule, it is not the thickness but the coating weight in g/m2 that is specified. Zinc phosphate coatings on steel can have a weight of 1 to 60 g/m2.
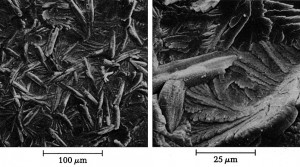 Relationships between weight per unit area and layer thickness of homogeneous layers can generally be established via the density of the layers. However, the densities of 3.04 g/cm3 for hopeite crystals, which characterize the phase composition of zinc phosphate layers, and 3.18 g/cm3 for huréaulite crystals, which build up the manganese phosphate layers, should only be expected for the layers in question if they consisted of this one phase, were covered without pores and if their surfaces were flat. Instead, as SEM images clearly show, the zinc and manganese phosphate layers consist of a crystal cluster which, depending on the various factors influencing the layer formation, always exhibits a certain porosity and, in some cases, considerable surface roughness.
Relationships between weight per unit area and layer thickness of homogeneous layers can generally be established via the density of the layers. However, the densities of 3.04 g/cm3 for hopeite crystals, which characterize the phase composition of zinc phosphate layers, and 3.18 g/cm3 for huréaulite crystals, which build up the manganese phosphate layers, should only be expected for the layers in question if they consisted of this one phase, were covered without pores and if their surfaces were flat. Instead, as SEM images clearly show, the zinc and manganese phosphate layers consist of a crystal cluster which, depending on the various factors influencing the layer formation, always exhibits a certain porosity and, in some cases, considerable surface roughness.
Due to the surface morphology of the phosphate layers, the apparent density, which is a measure of the space filling of the phosphate layers, can assume very different values between 0.9 and 2.5 g/cm3. The widespread view that it is always possible to assign certain lower apparent density ranges to thin phosphate layers and certain higher apparent density ranges to thick layers is incorrect. Despite the presence of thick phosphate layers with basis weights between 22 and 38 g/m2, average layer thicknesses between 14 and 25 μm, the calculated apparent densities show fluctuations between 0.9 and 2.07 g/cm3.
Due to the very different values that the apparent density of phosphate layers can assume even with layers of approximately the same basis weight, the conversion of basis weights into layer thicknesses, even when using an average value for the apparent density of zinc phosphate layers of approximately 1.7 g/cm3, leads to results that are generally subject to an error of up to ± 45 %. A conversion of basis weights into coating thicknesses can only lead to satisfactory results if reliable empirical values for the apparent densities are available, which were determined during the phosphating of a material of very similar composition and surface properties in a phosphating bath of the same composition under the same working conditions.
Coating thickness measurement
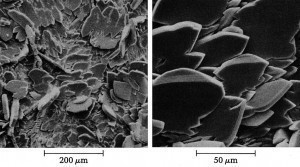 Phosphated surfaces have a sponge-like appearance when enlarged accordingly. This property is perfect for anchoring, e.g. with paintsThecross-section method (DIN 50950:1984-10) can also be used to measure the local layer thickness of phosphate layers. However, this is not used in practice. However, the method of removing part of the phosphate coating on the test piece and measuring the resulting gap between the phosphate coating and the metallic substrate using a scanning device to measure the surface roughness is used more frequently. For this purpose, part of the sample surface is covered with plastic adhesive tape or with a strippable coating that is resistant to the phosphate layer removal agent used. The phosphate layer is then removed down to the base metal using a stripping agent. After carefully removing the masking film, the sample surface is scanned perpendicular to the phosphate metal/bright metal boundary using a mechanical scanning device and recorded. The coating thickness can be read from the jump at the boundary line observed in the surface profilogram. This measuring method is not suitable for phosphate coatings whose thickness is in the range of the roughness of the sample surface, as is always the case with iron phosphate-iron oxide coatings.
Phosphated surfaces have a sponge-like appearance when enlarged accordingly. This property is perfect for anchoring, e.g. with paintsThecross-section method (DIN 50950:1984-10) can also be used to measure the local layer thickness of phosphate layers. However, this is not used in practice. However, the method of removing part of the phosphate coating on the test piece and measuring the resulting gap between the phosphate coating and the metallic substrate using a scanning device to measure the surface roughness is used more frequently. For this purpose, part of the sample surface is covered with plastic adhesive tape or with a strippable coating that is resistant to the phosphate layer removal agent used. The phosphate layer is then removed down to the base metal using a stripping agent. After carefully removing the masking film, the sample surface is scanned perpendicular to the phosphate metal/bright metal boundary using a mechanical scanning device and recorded. The coating thickness can be read from the jump at the boundary line observed in the surface profilogram. This measuring method is not suitable for phosphate coatings whose thickness is in the range of the roughness of the sample surface, as is always the case with iron phosphate-iron oxide coatings.
For the non-destructive measurement of the layer thickness of phosphate coatings on iron and steel, the reduction of the adhesive force of a permanent magnet (adhesive force principle), the influencing of the magnetic flux (magnetic-inductive method), the weakening of the induction of an eddy current generated by high-frequency alternating current in the base metal (eddy current method), the absorption or change in the intensity of the back radiation of electron beams (radiation measurement method) by the phosphate coatings can be used. The latter two methods also allow the thickness of phosphate coatings to be measured on non-ferromagnetic base metals (zinc, aluminum, etc.). With the exception of the radiation measurement methods, which have hardly ever been used for measuring the thickness of phosphate coatings, all other non-destructive measurement methods use probes to determine the coating thickness.
Literature
[1] DIN 50942:1996-09 - Phosphating of metals
[2] DIN 50950:1984-10 - Measurement of coating thicknesses
[3] Technology of electroplating, 2nd edition. Eugen G. Leuze Publishing House
[4] Die Phosphatierung von Metallen, 3rd edition. Eugen G. Leuze Publishing House
[5] On the characterization of the coating thickness of phosphate layers by specifying the weight per unit area and the layer thicknesses. Electroplating 63 (1972) 1, 11-23
[6] Coating thickness measurement, 2nd edition. Eugen G. Leuze Verlag, Saulgau, 1968
[7] Phosphating in Handbuch der Galvanotechnik, edited by Dettner, H. W, and Elze, J. Volume III, pages 86-123, Carl Hanser Verlag, Munich, 1969
[8] Bonder®-Technik No. 16 (May 1969): Chemical surface treatment prior to electrocoating
[9] Quantitative test for zinc phosphate coating quality. SAE Technical Paper Series No. 780 187 (1978)


