Chrome layers play a decisive role in the decorative and functional field of electroplating. The bright, shiny and hard layer is very important in the fittings, furniture and automotive industries. Replacing hexavalent chromium baths with trivalent electrolytes often results in the problem that the layers differ visually from one another, making mixed plating impossible. The Saphir 2000 BL process from Kiesow Oberflächenchemie GmbH & Co. KG in Detmold is a modified, trivalent chromium process which enables depositions with b* values in the negative range (-0.5 to -1). It has been specially developed for the requirements of the automotive industry (Fig. 1).
Saphir 2000 BL has been on the market for about a year and has proven to be a reliable and robust trivalent chromium process. It is characterized by the fact that it works reliably even under high loads and coating thicknesses of up to 0.3 µm can be achieved.
A major advantage is the simple waste water treatment. The Saphir 2000 BL electrolyte does not contain any complexing agents and can therefore be disposed of in a standard chemical-physical waste water treatment plant by means of simple neutralization treatment. The limit value for chromium (total) is 0.5 mg/L for waste water in accordance with Annex 40 AbwV and can therefore be safely and easily complied with.
In contrast to electrolytes with strong complexing agents and correspondingly high requirements for complex treatment, the simple handling of wastewater from Saphir 2000 BL processes was also confirmed by the bi.bra Abwassertechnik GmbH team. In the tests carried out, the electrolyte was diluted to varying degrees (1:40, 1:20 and 1:10) in order to subsequently carry out neutralization precipitation (Table 1).
|
Step |
Action |
Value |
Chemicals |
Remark |
|
1 |
Acidify |
pH 1.5 |
HCl |
|
|
2 |
Reaction time |
30 min |
||
|
3 |
Neutralization |
pH 9.5 |
Ca(OH)2 |
|
|
4 |
Reaction time |
60 min. |
||
|
5 |
Flocculation |
bi.bra Floc-MK |
||
|
6 |
Filtration |
Pleated filter |
||
|
7 |
Clear filtrate measurement |
 Fig. 2: Precipitation of the chromium hydroxide after the addition of milk of lime (a); filtration of the solution to separate the chromium hydroxide from the clear phase (b); collection of the clear phase for analysis (c)All samples were then passed separately through a pleated filter and the clear filtrate was collected (Fig. 2). The clear filtrate was analyzed in accordance with DIN EN ISO 11885; 09-2009. It was found that the Cr concentration in the wastewater was below 0.3 mg/L Cr for all samples and thus the discharge conditions were met.
Fig. 2: Precipitation of the chromium hydroxide after the addition of milk of lime (a); filtration of the solution to separate the chromium hydroxide from the clear phase (b); collection of the clear phase for analysis (c)All samples were then passed separately through a pleated filter and the clear filtrate was collected (Fig. 2). The clear filtrate was analyzed in accordance with DIN EN ISO 11885; 09-2009. It was found that the Cr concentration in the wastewater was below 0.3 mg/L Cr for all samples and thus the discharge conditions were met.
Laboratory report at:
www.kiesow.org/aktuelles/saphir-2000-bl-248
In production operations, the use of an ion exchanger system (Fig. 3) is necessary for bath maintenance in order to safely remove foreign metals such as Cu, Ni, Fe, Zn from the electrolyte using special ion exchange resin, depending on the upstream processes. Depending on the production volume, this treatment system can be designed with one or two lines or with manual or automatic control.
Overall, the separations of the Saphir 2000 BL electrolyte are comparable to those of hexavalent systems, and waste water treatment does not present any difficulties. It is a user-friendly electrolyte that fully meets the requirements of a decorative chrome layer.
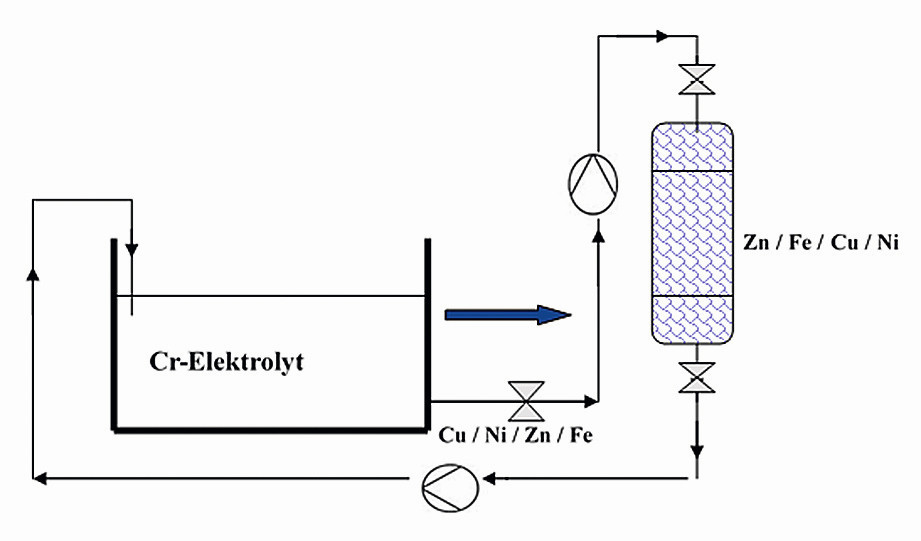 Fig. 3: Schematic representation of the chrome system with connected ion exchanger system
Fig. 3: Schematic representation of the chrome system with connected ion exchanger system
We would welcome the opportunity to introduce you to the Saphir 2000 BL electrolyte. Our technical field service will be happy to advise you during the introductory phase of the Saphir 2000 BL process.
THE AUTHORS
Dr. Reiner Dickbreder, Laboratory Manager/Development,
Lisa Büker and Martin Bertels,
Research and Development,
Kiesow Oberflächenchemie GmbH & Co KG,
Detmold


