A different kind of surface reaction: Empa researchers allow coupling reactions to take place on a gold surface in a high vacuum rather than in liquid. The findings could also be used to further develop liquid chemistry.
Chemical syntheses in liquids and gases take place in three-dimensional space. Something new has to emerge from random collisions in an extremely short time. But there is another way: on a gold surface in an ultra-high vacuum, molecules that are still next to each other can be made to combine - even those that would never want to react with each other in liquids. Researchers at the Swiss Federal Laboratories for Materials Science and Technology Empa have now discovered such a reaction. The best thing about it: the experts can "switch to slow motion" and watch every step of the reaction (Fig. 1). In chemistry, there are structures that are particularly stable, such as the so-called "benzene ring" consisting of six carbon atoms linked together. Such rings form the structural basis for graphite and graphene, but they are also found in many dyes - such as the denim dye indigo and in many medicines such as aspirin. When chemists want to specifically build up such rings, they use so-called coupling reactions, which usually bear the name of their inventors: for example, the Diels-Alder reaction, the Ullmann reaction, Bergman cyclization or the Suzuki coupling. Now there is another one that does not yet have a name. It was discovered by a team from Empa together with the Max Planck Institute for Polymer Research in Mainz.
Everything in the dry
The Empa researchers dispensed with the liquids in their chemical synthesis and instead attached the starting materials to a gold surface in an ultra-high vacuum. The starting material (diisopropyl-p-terphenyl) can be viewed again at leisure in the low-temperature scanning tunneling microscope before the researchers turn up the heating.
Turning up the heat - movement on the dance floor
Nothing happens at room temperature, but at around 200 °C an amazing reaction takes place that would never happen in liquids: the two isopropyl groups - normally completely harmless chemically - combine to form a benzene ring. The reason: due to the firm "assembly" on the gold surface, a hydrogen atom is first loosened and then released from the molecule. This creates carbon radicals that are waiting for new partners. And there are many partners on the gold surface. At 200 °C, the molecules vibrate and perform rapid pirouettes - there is a lot of movement on the golden dance floor. Soon, what belongs together comes together (Fig. 2).
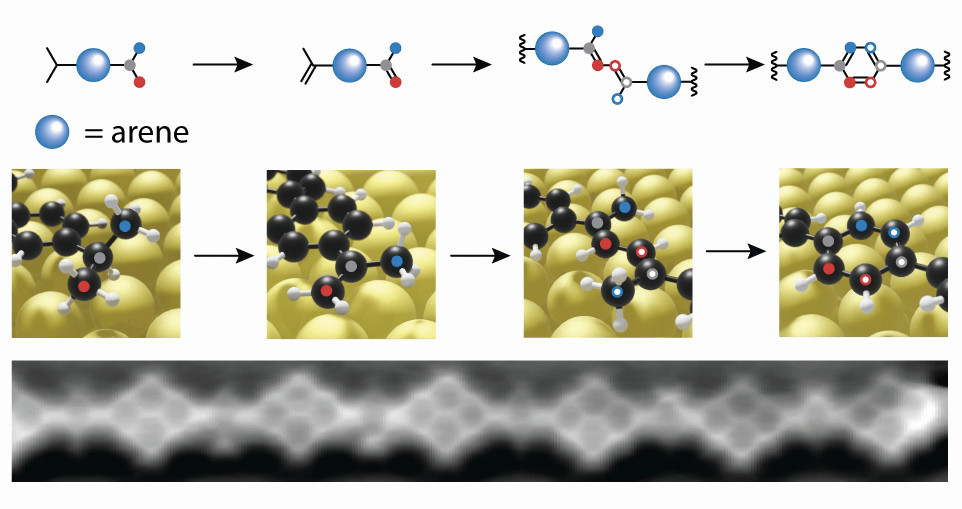 Fig. 2: This is how chemical synthesis works on the surface: a hydrogen is abstracted from saturated isopropyl groups. At 200 °C, the carbon atoms (red and blue in the picture above) combine to form a new benzene ring. This is how individual molecular building blocks become a polymer chain - visible in the atomic force microscope (bottom)
Fig. 2: This is how chemical synthesis works on the surface: a hydrogen is abstracted from saturated isopropyl groups. At 200 °C, the carbon atoms (red and blue in the picture above) combine to form a new benzene ring. This is how individual molecular building blocks become a polymer chain - visible in the atomic force microscope (bottom)
And once again in slow motion
The coupling on the golden surface has two advantages. Firstly, no constraint is necessary: the reaction takes place without mediating boric acids or halogen atoms flying away. It is a compound involving only saturated hydrocarbons. The starting materials are cheap and easy to obtain, and there are no toxic by-products.
The second advantage is that the researchers can watch every step of the reaction - something that is also not possible with classic "liquid" chemistry. The Empa team simply turns up the heating of the gold surface step by step. At 180 °C, the molecules have only connected one arm with its neighbors, the second still protrudes freely into the dance floor. If the gold surface is now cooled down in a scanning tunneling microscope, the molecules can be viewed and "photographed" shortly before "marriage". This is exactly what the researchers have done. In this way, the reaction mechanism can be followed in the form of "snapshots".
Opportunities for a "new" chemistry
The researchers and their colleagues expect two effects to emerge from the current work. Firstly, the "snapshot method" could also be suitable for elucidating completely different reaction mechanisms. At Empa, devices are being developed that can elucidate such chemical reactions step by step using ultra-short laser pulses in a scanning tunneling microscope. This could provide additional insights into chemical reactions and soon shake up some old theories.
However, the research results "from the dry" could also be useful for the further development of "liquid" chemistry. Up to now, most of the reactions documented in the literature have come from classical liquid chemistry and the microscope researchers have been able to recreate these experiments. In the future, certain reactions could also be designed in the scanning tunneling microscope and later transferred to liquid or gaseous chemistry.


