Chemical conversion coatings are one of the most common surface modification techniques that provide a barrier between metal and its surrounding environment [1]. The treatment can be carried out by dipping, spraying or by application of brush. The term chemical conversion is used where the exposed metal surface gets converted into the chemically inert inorganic coating by a chemical or electrochemical process. The coatings, in addition to corrosion protection, may impart requisite functional properties, enhanced surface hardness and a good base for application of subsequent paints, lubricants, adhesives, etc. The properties of these coatings depend on the kind of substrate metal, the composition and structure of the coatings. On the other hand, the composition and structure of the coatings depend on the bath composition and operating parameters of the process.
2.1 Black Chromate Conversion Coatings
The chromate conversion coatings are formed because the metal surface dissolve to a small extent, causing a raise in pH value at metal surface-solution interface [2]. A thin film of metal chromate gets precipitated on the metal substrate. The film is soft and gelatinous when freshly formed, once dried it slowly attains enough hardness. The freshly treated works should therefore be handled very carefully. The chromate coating offers protection to metallic substrate through two mechanisms: (i) provides a non-reactive barrier to humidity and air, thus retarding the corrosion; (ii) the film retains a water absorbing characteristic as long as it remains in a hydrated form. When scratched or mechanically damaged enough water is absorbed by the film to swell and mend at the damaged areas. While the heating below 75 °C is benefited in hastening the hardening process, prolong heating above these temperatures may result in excessive dehydration of film that may result in reduction of its protection value.
Formation of black chromate conversion coatings on zinc-iron (0.58 wt.%) alloy was reported [3, 4]. The coatings were deposited by immersion in a proprietary acid sulphate chromate bath consisting of CuSO4.5H2O: 30 g/L, CrO3: 20 g/L, NaCOOH2.H2O: 15 g/L, addition agent: 70 ml/L, accelerator: 0.2 g/L and inhibitor: 0.30 g/L operating at room temperature, pH: 1-2. The bath was adjusted so as to achieve a desired composition of 0.58 % Fe which provides the optimum corrosion performance. Post-deposition, the coatings were dried in air for 24 hours.
XPS measurements confirmed Zn, Cr and O, as the major constituents of the chromate conversion coating and a small amount of Cu, Ag, S and Fe was also detected. The coating showed a two-layers structure. The outer layer that made the major part of the coating consisted of Cr2O3, Cr(OH)3, Cr(OH)CrO4, Zn2(OH)2CrO4 and a small amount of absorbed water. The overall electrochemical reaction may be described as follows:
3 Zn + Cr2O72– + 14H+ → 3 Zn2+ + 2 Cr3+ + 7H2O
3 Zn + 2 CrO42– + 16 H+ → 3 Zn2+ + 2 Cr3+ + 8 H2O
Cr2O72– + 2 OH– → 2 CrO42– + H2O
Cr3+ + OH– + CrO42– → Cr(OH)CrO4
2 Zn2+ + 2 OH– + CrO42– → Zn2(OH)2CrO4
2 Cr3+ + 6 OH– → Cr2O3. 3H2O
The inner layer was a transition region where the content of metallic zinc increased and that of metallic chromium and oxygen decreased until a constant composition was reached. Fe was incorporated into the inner layer of chromate conversion coating. Chemical analysis showed that the chromium content of the coating was the sum of Cr(III), 0.0295 g/m2 and Cr(VI), 0.02 g/m2. The coatings exhibited lustrous black appearance due to the incorporation of small amount of cuprous oxide (Cu2O), silver oxide (Ag2O), and ferrous chromate (FeCrO4) along with high concentration of hexavalent chromium on the coating surface. The surface morphology of the coating was characterized by dried riverbed’ microcracks. Although the concentration of chromate in solution was low, the coatings showed good corrosion resistance due to their good ''self-healing’’ ability. The properties of black chromate conversion coatings on electrodeposited zinc and zinc-cobalt alloy have been investigated by Nikolova et al. [5]. It has been established that the presence of certain additives, e.g., ethoxylated aliphatic alcohols C12-C14 with 18 ethoxy groups and Na2SiO3 affect the absorption properties and thickness of the film. The inclusion of small amounts (up to 1 wt.%) of cobalt in the zinc sublayer strongly influence the chromate coating properties. Black coatings formed on Zn and Zn-Co alloys attain the values of integral coefficient of solar absorption as 0.88–0.90 and 0.90–0.93, respectively. A method for the determination of the optical thickness of the coating was also described.
2.2 Black Chrome-Manganese Conversion Coatings
A dark brown to black colour chrome-manganese conversion coating can be obtained on all types of magnesium alloys. These coatings provide the moderate corrosion resistance by itself without application of subsequent paint. The treatment is usually carried out after solvent degreasing, alkaline cleaning and acid pickling. Instead of acid pickling, a mild acid cleaning in chromic acid is recommended for components of close dimension tolerance. The following bath formulation is used for chrome-manganese conversion coating [6]:
Sodium or potassium dichromate,
Na2Cr2O7.2H2O or K2Cr2O7 80–100 g/L
Manganous sulphate, MnSO4.H2O 40–50 g/L
Magnesium sulphate, MgSO4.7H2O 40–50 g/L
pH 4.0
Temperature Room temperature to boiling
Tank material SS, PP
Time 15 minutes to 2 hours as shown
below for depending on operating bath temperatures
Room temperature 2 hours
50–60 °C 45 minutes
60–80 °C 30 minutes
Boiling 15 minutes
Coating thickness 7–11 µm
Film microhardness improves when baths are operated at higher temperatures. As the solution is used, its pH is increased from 4.0 to 5.0. Then the solution has to be replenished with the addition of 50 g/L of manganese sulphate and the pH has to be adjusted back to 4.0 with the addition of a solution of 250 g/L sulphuric acid and 250 g/L chromic acid. Addition of 1 ml/L of the solution, reduces the pH of 1 L bath by 0.1.
A process of black chromate conversion coating on magnesium-lithium (LA141A and MLA 9, Al 1.25 %, Li 10%, by weight, balance Mg) and magnesium-aluminium alloys (such as AZ91, AZ80, and AM60) was described by Sharma [7–9]. The process involves the following sequence:
(a) Ultrasonic solvent degreasing in isopropanol for 3–5 minutes.
(b) Alkaline cleaning in sodium hydroxide 55 g/L, trisodium orthophosphate 10 g/L and potassium fluoride 1 g/L operating at 85–90 °C for 5–6 minutes, followed by water rinsing.
(c) Acid cleaning in 50 % chromic acid for 4–7 minutes for Mg-Li alloys. For Mg-Al alloys a solution of chromic acid 120 g/L and nitric 70 % 110 ml/L for 30–60 seconds was used.
(d) Chemical brightening in a solution containing 8–10 % orthophosphoric acid (V/V) in isopropanol for 3–5 minutes, followed by rinse in isopropanol, dip in hot water and dry for Mg-Li alloys. For Mg-Al alloys a fluoride activation in 40 % HF 400 ml/L for 1 minute was carried out.
(e) Chromate conversion coating
Potassium dichromate, K2Cr2O7 90 g/L
Manganous sulphate, MnSO4.H2O 40 g/L
Magnesium sulphate, MgSO4.7H2O 40 g/L
Potassium fluoride, KF 1–2 g/L
Temperature 55–90 °C
Time 2-3 hours depending on the bath
temperature, followed by water rinsing;
Tank material SS 304 with immersion heaters
Coating thickness 8–11 µm
(f) Sealing only for magnesium-lithium alloys in a solution containing 2 % potassium dichromate, K2Cr2O7 and 10 % ammonium bifluoride, (NH4)HF2 at room temperature for 3 minutes, hold over an open vessel of hot water (70–75 °C) for 2–3 hours.
(g) Heat treatment by placing the job over glass or aluminium plate in an oven operating at 70–75 °C for 1–2 hours for magnesium-aluminium alloys and for 6 hours for magnesium-lithium alloys.
(h) Rinse in hot water for 2–3 minutes, polish gently with soft cloth to remove the boom, dip in hot isopropanol and dry.
The black chromate film described herein provide a high solar absorptance (0.91) and infrared emittance (0.90) making them suitable for spacecraft thermal control application.
2.3 Galvanic Black Anodizing
Galvanic black anodising on magnesium alloys is an improved version of chromate conversion coating. Processes of galvanic black anodising were investigated in details by Sharma et al. [10–16] on magnesium alloys AZ31B, ZM21 and Mg-Li alloys. Following sequence of operations was used:
(a) Solvent degreasing in isopropyl alcohol for 5–10 minutes.
(b) Alkaline cleaning in a solution containing sodium hydroxide 50 g/L and trisodium orthophosphate 10 g/L; operating at 60 ± 5 °C for 5–10 minutes. Followed by water rinsing.
(c) Acid pickling in a solution containing:
For AZ31B alloy:
chromium trioxide 180 g/L, ferric nitrate 40 g/L and potassium fluoride 3.75 g/L for 2 minutes; followed by water rinsing.
For ZK 60A alloy:
(a) 50 % chromium trioxide for 1–2 minutes; water rinsing. (b) chemical brightening in a solution containing chromium trioxide 180 g/L, ferric nitrate 40 g/L and potassium fluoride 3.75 g/L for 10–15 seconds; followed by water rinsing. This acid pickling process provides effective cleaning without significant attack on base metal and hence is recommended for close dimensional tolerance components.
For Mg-Li alloys:
(a). chromium trioxide 500 g/L, ferric nitrate 1 g/L and potassium fluoride 0.5–1.0 g/L for 3–5 minutes with a post treatment of water rinse.
(b) fluoride activation by dipping in 40 % hydrofluoric acid (50 ml/L) for 10 minutes, followed by water rinsing.
(d) Black anodizing in the following optimal conditions:
Potassium dichromate, K2Cr2O7 25 g/L
Ammonium sulphate, (NH4)2SO4 25 g/L
pH 5.5, (5.8 for ZM21)
Temperature 25 ± 2 °C (room temperature)
Time 60 minutes
Cathode anodizing tank (SS)
Anode to cathode area ratio 1:5 to 1:10
Galvanic current/voltage 0.8–2.4 mAcm-2 / 1.2–3.6 mVcm-2
Coating thickness 12–15 µm
Post treatment water rinse
The process yielded satisfactory coatings in a wide range of electrolyte pH (4.5–6.5) and concentration (20–30 g/L of each constituent).
(e) Heat treatment at 70 °C for 2 hours in an electric oven with clean hot air circulation.
The process can be represented by the following equations.
Electrolytic reaction (ionisation):
K2Cr2O7 → 2K+ + Cr2O72–
(NH4)2SO4 → 2NH4+ +SO42–
H2O → 2H+ + OH–
Anodic reaction:
Mg0 → Mg2+ +2e–
Mg2+ + Cr2O72– + 6 OH– / 3 SO42– → 2 MgCrO4 + 2Cr(OH)3 + Cr2(SO4)3 + 3O2 + 6 e–
Cathodic reaction:
K+ + OH– → KOH
NH4+ + 4 OH– → NH4OH
2 H+ + 2e– → H2↑
![Fig. 2.1: Scanning Electron Micrographs of galvanic black anodic film on Mg-Li alloy [10] Fig. 2.1: Scanning Electron Micrographs of galvanic black anodic film on Mg-Li alloy [10]](/images/stories/Abo-2021-07/thumbnails/thumb_gt-2021-07-0015.jpg) Fig. 2.1: Scanning Electron Micrographs of galvanic black anodic film on Mg-Li alloy [10]The word galvanic implies that the process does not require any outside current for electrolysis. When the job is immersed in the anodising solution and is connected to the stainless-steel anodising tank, a flow of current starts from job (anode) to tank (cathode), due to the dissolution of the outer surface of the alloy, which sustains the reaction. Thus, the galvanic coating is the product of a controlled reduction-oxidation process.
Fig. 2.1: Scanning Electron Micrographs of galvanic black anodic film on Mg-Li alloy [10]The word galvanic implies that the process does not require any outside current for electrolysis. When the job is immersed in the anodising solution and is connected to the stainless-steel anodising tank, a flow of current starts from job (anode) to tank (cathode), due to the dissolution of the outer surface of the alloy, which sustains the reaction. Thus, the galvanic coating is the product of a controlled reduction-oxidation process.
Coatings of this type have a gel-like structure as formed, but harden after drying and form a microcracked 'mud-crack’ pattern. After heat treatment these microcracks became wider and edge curling of the blocks of the coating occurred. This probably resulted due to the dehydration and shrinkage of the film. The term micro-crack refers to a crack that does not extend from the base metal to the surface of the deposit. The hardness of the anodic coating was found to increase after sealing and by post deposition heat treatment. Scanning Electron Micrographs (SEM) of galvanic black anodic film on Mg-Li alloy is shown in Figure 2.1.
The process is simple and economical; bath operates at the room temperature and no external current is required. The deposits were characterized by optical and scanning electron microscopy, adhesion tests, corrosion studies, thickness measurement and microhardness evaluation. These deep black colour coatings provide high solar absorptance and thermal emittance (> 0.90) values. The space worthiness of the coatings was evaluated by the humidity, the thermal cycling and the thermovacuum tests and measurement of optical properties.
2.4 Black Molybdate / Permanganate Coatings
Molybdates (MoO42–) have long been utilised as one of the green corrosion inhibitors and are analogous to chromates (CrO42–), molybdenum and chromium being from the same group (VI A) of the periodic table [17–19]. The mechanism of molybdate conversion coatings is similar to formation of chromate coating. The substrate metal cation say aluminium (Al3+) may combine with molybdate anions to form aluminium molybdenum oxide, [Al2(MoO4)3].
A black galvanic molybdate conversion coating process on magnesium alloy AZ31B was reported by Parida et al. [20] for spacecraft thermal control application. The following sequence of operations was adopted:
(a) Mechanical cleaning with 600 grade silicon carbide paper.
(b) Solvent degreasing in isopropanol for 5–10 minutes.
(c) Alkaline cleaning in a solution of sodium hydroxide 50 g/L and trisodium orthophosphate 10 g/L at 65 ± 5 °C for 5–6 minutes, followed by water rinsing.
(d) Acid cleaning in chromic acid 180 g/L and ferric nitrate 40 g/L at 25 ± 5 °C for 5–10 minutes; water rinsing.
(e) Molybdate conversion coating in a following bath:
Ammonium molybdate, (NH4)6MO7O24.4H2O 20 g/L
Magnesium chloride, MgCl2.6H2O 1 g/L
pH 5.5
Temperature 25 ± 5 °C (room temperature)
Time 60 minutes
Cathode SS 304 tank
Galvanic current/voltage 0.8–2.4 mAcm-2 / 1.3–3.8 mVcm-2
Coating thickness 12–15 µm
Post treatment water rinse
Jobs are connected to the SS 304 container to form a galvanic cell. The elemental composition, surface morphology, environmental and thermal stability of coatings were studied. Energy dispersive X-ray (EDX) and infrared spectral studies reveal that these coatings consist of hydroxides and oxides of molybdenum and water of hydration. Scanning electron microscopy studies revealed the river bed type appearance of the coating’s morphology. The thermoanalytical investigations of coating have been carried out using thermogravimetry (TG), derivative thermogravimetry (DTG) and differential scanning calorimetry (DSC). Thermal stability studies revealed two major changes (i) dehydration started at ~50 °C and continued until ~250 °C, and (ii) decomposition of molybdenum hydroxides thereafter. The black molybdate conversion coatings provide high absorptance (0.90) as well as high emittance (0.89), and excellent environment stability for stringent space conditions.
Gabe and Gould [21] made detailed studies on the optimization of black molybdate conversion coating process. Both chemical and electrochemical treatments have been explored and influence of various process parameters (pH, concentration, time, temperature etc.) have been examined. The authors have made the following observations:
(I) Black molybdate coatings can form on tin, zinc and hot dipped galvanized steel surfaces; those on aluminium were distinctly inferior.
(II) Optimum process conditions include a solution containing 30 g/L Na2MoO4 at pH 3–5 for 5–10 minutes. Immersion treatments or cathodic potentiostatic polarization (320 mV overpotential) can be employed.
(III) Coatings produced are flat absorber with solar absorptances of 99 % and thermal emittances of 80 %. Solar selectivity of about 2.5 (α = 0.98 and ε = 0.4) can be achieved with 2 minutes immersion time, where coatings are thin and incomplete.
(IV) Coatings on hot dipped galvanized steel surfaces require longer processing times to produce equivalent coatings than on wrought zinc foil, and a grain etch effect may be noticeable.
A process of black molybdates coatings on aluminium alloys has been described by Uma Rani et al. [22]. The process imparts satisfactory black coating with excellent environment stability. A 5 ± 1 µm thick coating bestows an infrared emittance ~ 0.70, and solar absorptance > 0.92. After following a suitable cleaning cycle of substrate, the coatings were obtained using the following process sequence:
(a) Zincating by immersion in a solution containing nickel sulphate (28–32 g/L), zinc sulphate (35–45 g/L), sodium hydroxide (100–110 g/L), potassium cyanide (8–12 g/L), potassium bitartarate (35–45 g/L), copper sulphate (4–6 g/L) and ferric chloride (1 g/L) at room temperature for 1 minute; followed by water rinsing.
(b) Stripping the first zinc layer by immersion in 50 % nitric acid solution at room temperature for 1–2 minutes, water rinsing.
(c) Re-zincating by repeating the above zincating step for 5 minutes, water rinsing.
(d) Black molybdate coating in a solution containing ammonium molybdate (50 g/L), ammonium chloride (30 g/L), boric acid (7.5 g/L) and nitric acid (70 %, 5–7 ml/L), pH 3.3 ± 0.2 at room temperature for 1 hour with mild agitation, water rinsing.
The permanganate being an oxidising agent have the potential to produce a passivating film of residual permanganate on the metal surfaces. The black permanganate coating can be formed on the zincated aluminium alloys [23]. The zincated layer is first formed as described above [22] and is oxidised in a permanganate solution consisting of potassium permanganate 15 g/L, nitric acid (70 %) 4 ml/L and copper nitrate 25 g/L at pH 3.3 ± 0.2, 50 °C for 30 minutes. To enhance the colour, the coating is further oxidised in arsenic trioxide solution (5 g/L) at room temperature at a pH of ~2.0 for 15 seconds. This is followed by sealing in boiling water for 30 minutes. Under optimal conditions, the process yields a satisfactory black coating where a 6 µm coating provide a solar absorptance of 0.90 and IR emittance of 0.80. Zhang et al. [24] described a process of phosphate–permanganate conversion coating on magnesium-lithium alloy. The coatings were obtained with a solution consisting of potassium permanganate: 40 g/L and potassium dihydrogen phosphate: 50 g/L; pH 4.5, temperature: 50 ± 5 °C; time: 60 minutes; followed by water rinsing. The morphology, the composition and the corrosion resistance of the coating were examined. A thin and non-penetrating cracked morphology was reported. The main elements of the conversion coating were Mg, O, K, P and Mn. The results of the electrochemical measurements and the immersion tests demonstrated that the corrosion resistance of the magnesium-lithium alloy has been improved by the phosphate-permanganate conversion treatment.
2.5 Chemical Blackening or Black Oxide Coating
Formation of black oxide coating or chemical blackening is a conversion coating process on ferrous materials, stainless steel, copper and zinc-based alloys. It usually provides a mild corrosion protection. To further improve its corrosion resistance, the black oxide must be impregnated with oil or wax. One of the advantages of chemical blackening over other coatings is its minimal dimensional build-up.
On steel a standard black oxide coating of magnetite (Fe3O4) is formed, which is mechanically more stable on the surface and provides better corrosion protection than red oxide (rust), Fe2O3. Hot alkaline nitrate black oxidizing process provides good results [25–31]. Though the mid temperature (104–118 °C) process can also be used. However, it offers relatively very poor abrasion resistance and corrosion protection than the hot blackening process and hence is good only for architectural finishes and not recommended for engineering applications.
Prior to blackening the parts are pre-treated (a) solvent degreasing in trichloroethylene, (b) acid cleaning in a solution containing nitric acid (70 %) 275–325 ml/L, hydrofluoric acid (40 %) 60–80 ml/L, ammonium bifluoride 55–60 g/L, at 50 ± 5 °C for 10–20 minutes.
Black oxide coating on stainless steel 300 and 400 series and the precipitation-hardened 17-4 pH stainless steel can be carried out in a mixture of sodium hydroxide (66 %), oxidizing salts (nitrates and nitrites, 33 %), and some sulphur salts, operating at boiling temperatures- 130 to 150 °C (141 °C) for 5–20 minutes. The baths are prepared by adding salt mixture to sufficient water to adjust the boiling point of the solution to the desired operating temperature within the given range.
Water must be periodically added to the bath, with proper controls to prevent a steam explosion. This solution can also be used for blackening of cast iron and mild low-carbon steel. The black oxide is more dense than red oxide, but it is porous when freshly formed, so oil can be applied on the heated part, which seals it by “sinking” into it. The combination prevents corrosion of the workpiece.
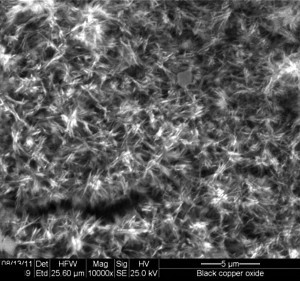 Fig. 2.2: Fibrous structure of black copper oxide coatingIn an attempt to reduce the operating temperature of blackening solution, Eckl et al. [32] have investigated an electrochemical route for nitrite free hot alkaline blackening of steel. A 30 g sodium hydroxide or blackening salt was dissolved in 30 mL water and the blackening was carried out electrochemically by applying a potential in the range of 0 to 0.6 V vs. Pt at 80–120 °C. A further decrease in the bath temperature with simultaneous increase of potential did not yield the desired result due to the formation of Ferrate (VI) ions {[FeO4]2−}, and the slow kinetics of the Schikorr reaction. The optimal results were obtained with 23 M sodium hydroxide solution at an electrode potential of 0.5–0.6 V at 80 °C for 12 minutes. Cyclic voltammetry, atomic force microscopy, scanning electron microscopy and auger electron spectroscopy were used to characterize the electrochemically blackened steel surfaces.
Fig. 2.2: Fibrous structure of black copper oxide coatingIn an attempt to reduce the operating temperature of blackening solution, Eckl et al. [32] have investigated an electrochemical route for nitrite free hot alkaline blackening of steel. A 30 g sodium hydroxide or blackening salt was dissolved in 30 mL water and the blackening was carried out electrochemically by applying a potential in the range of 0 to 0.6 V vs. Pt at 80–120 °C. A further decrease in the bath temperature with simultaneous increase of potential did not yield the desired result due to the formation of Ferrate (VI) ions {[FeO4]2−}, and the slow kinetics of the Schikorr reaction. The optimal results were obtained with 23 M sodium hydroxide solution at an electrode potential of 0.5–0.6 V at 80 °C for 12 minutes. Cyclic voltammetry, atomic force microscopy, scanning electron microscopy and auger electron spectroscopy were used to characterize the electrochemically blackened steel surfaces.
The surface of copper and its alloys that have over 65 % coppers can be converted to black cupric oxide. The finished coating is adherent and chemically stable up to 200 °C; above this temperature it degrades due to oxidation of the base copper. To further increase the corrosion resistance, the oxide surface may be oiled, lacquered, or waxed. The process is commonly applied on copper alloy components and to stack multiple PCB’s for enhancing their heat radiation characteristics. It is also used as a pre-treatment for painting or enamelling. A pre-treatment of parts consisting of (a) solvent cleaning in trichloroethylene, (b) acid cleaning in 10 % hydrochloric acid (SG 1.18) for 15–20 seconds at room temperature, is recommended. The following are some typical bath formulations for copper blackening [33]:
(a) Sodium hydroxide: 30 g/L, sodium chlorite: 30 g/L, pH: 12.7-13.0, temperature: 65–70 °C, time: 30–40 minutes.
(b) 100–200 g/L of the mixture containing sodium hydroxide: 50 %–67 % and sodium chlorite: 33–50 %, temperature: 99–102 °C, time: 5–10 minutes.
(c) Trisodium phosphate: 100 g/L, sodium hydroxide: 50 g/L, sodium chlorite: 30 g/L; temperature: 95 °C, time: 15 minutes.
(d) Trisodium phosphate: 5–15 g/L, sodium hydroxide: 10–20 g/L, sodium chlorite: 30–90 g/L, temperature: 65–95 °C, time: 2–5 minutes
(e) Electrochemical method (anodizing) in a solution of sodium hydroxide, 160 g/L, temperature: 90 °C, current density: 1.43 A/dm2, time: 2–10 minutes.
The oxide layer has a fibrous microstructure consisting of numerous dendrites of copper oxide crystals significantly increasing the bonding area between the copper surface and the polymer, resin. Due to such structure the black copper oxide coating has a velvet appearance (Figure 2.2). When a liquid resin is applied over the substrate surface it penetrates into the spaces between the dendrites and get interlocked in the oxide structure, which results in a strong adhesion. The coating formed with bath (a) above provides a solar absorptance of the order of 0.90–0.95 while the IR emittance is in the range of 0.30–0.40.
REFERENCES
[1] A.E. Hughes: Conversion Coatings. Corrosion and Prevention, Encyclopaedia of Interfacial Chemistry, Klaus Wandelt (Editor-in-Chief), 6.1(2018), 108–114, Doi: 10.1016/B978-0-12-409547-2.13441-9
[2]. Jeck Kinndle: Chromate conversion coatings, American Electroplaters Society, Inc, FL, (1986)
[3] M.P. Gigandet; J. Faucheu; M. Tachez: Formation of black chromate conversion coatings on pure and zinc alloy electrolytic deposits: role of the main constituents, Surf. Coat. Technol., 89(1997)3, 285–391, Doi:10.1016/S0257-8972(96)03013-7
[4] Z.L. Long, Y.C. Zhou, L. Xiao: Characterization of black chromate conversion coating on the electrodeposited zinc-iron alloy, Appl. Surf. Sci., 218(2003), 123–136, Doi: :10.1016/S0169-4332(03)00572-5
[5] M. Nikolova; O. Harizanov; P. Steftchef; I. Kristev; S. Rashkov: Black chromate conversion coatings on electrodeposited zinc, Surf. Coat. Technol., 34(1988), 501–514, Doi: 10.1016/0257-8972(88)90105-3
[6] A.K. Sharma: Chemical Conversion Coatings on Magnesium Alloys (Part 2), Galvanotechnik,118(2020)7, 1044–1050
[7] A.K. Sharma: Chromate conversion coatings for magnesium-lithium alloys, Met. Finish., 87(1989)2, 73–74
[8]. A.K. Sharma: A process of chromate coating on magnesium-lithium alloys, Indian Patent 170, 666, (1988)
[9]. A.K. Sharma: A process of flat absorber black chromate conversion coating on Mg-Al alloys, Indian Patent 180, 666, (1992)
[10] A.K. Sharma; R. Uma Rani; H. Bhojaraj; H. Narayanamurthy: Galvanic black anodizing on Mg-Li alloys, J Appl. Electrochem., 23(1993) 500–507, Doi: 10.1007/BF00707629
[11]. A.K. Sharma: Integral black anodizing on magnesium alloys, Met. Finish., 91 (1993)6, 57–63
[12]. A.K. Sharma; R. Uma Rani; A. Malek; K. S. N. Acharya; M. Muddu; S. Kumar: Black anodizing of a magnesium-lithium alloy, Met. Finish., 94(1996)4, 16–27, Doi: /10.1016/0026-0576(96)81263-3
[13]. A.K. Sharma: Galvanic black anodizing on magnesium alloy ZK 60A for thermal control applications, J Spacecraft Technol., 7(1997)1, 49–57
[14]. A.K. Sharma, R. Uma Rani, S.M. Mayanna: Thermal studies on electrodeposited black oxide coating on magnesium alloys, Thermochim. Acta, 376(2001) 67–75, Doi: 10.1016/S0040-6031(01)00534-2
[15]. A.K. Sharma: A process of integral black anodizing on magnesium alloys Indian Patent 179,637, (1991)
[16]. A.K. Sharma: A process of galvanic black anodizing on magnesium-lithium alloy substrates, Indian Patent 179,671, (1991)
[17] K. Sugimoto; Y. Sawada: The Role of Alloyed Molybdenum in Austenitic Stainless Steels in the Inhibition of Pitting in Neutral Halide Solutions, Corros., 32(1976)9, 347–352, Doi: 10.5006/0010-9312-32.9.347
[18] M. Urgen; A.F. Cakir, The effect of molybdate ions on the temperature dependent pitting potential of austenitic stainless steels in neutral chloride solutions, Corros. Sci., 32(1991)8, 835–852, Doi: 10.1016/0010-938X(91)90028-N
[19] G. Ruijini; M.B. Ives: The influence of addition of molybdate ions on pit growth in UNS S30100 stainless steel in chloride solution, Corros. 45(1989)7, 572–574, Doi: 10.5006/1.3577874
[20] B. Parida; R. Uma Rani; A.K. Sharma: Studies on chrome free galvanic conversion coating on Magnesium alloy AZ31B for space applications, Surf. Eng., 26(2010)5, 361–366, Doi: 10.1179/174329409X446340
[21] D.R. Gabe; S.E. Gould: Black molybdate conversion coatings, Surf. Coat. Technol., 35(1988)1-2, 79–91, Doi: 10.1016/0257-8972(88)90059-X
[22] R. Uma Rani; A.K. Sharma: Studies on black molybdate conversion coatings on aluminum, Galvanotechnik, 93(2002)7, 1736–1746+vi
[23] R. Uma Rani; A.K. Sharma; S.M. Mayanna; H. Bhojraj; D.R. Bhandari: Black permanganate conversion coatings on aluminium alloys for thermal control of spacecraft, Surf. Eng., 21(2005)3, 198–203, Doi: 10.1179/174329405X50019
[24]. H. Zhang; G. Yao; S. Wang; Y. Liu; H. Luo: A chrome-free conversion coating for magnesium–lithium alloy by a phosphate-permanganate solution, Surf. Coat. Technol., 202(2008), 1825–1830, Doi: 10.1016/j.surfcoat.2007.07.094
[25] Mitchell, A.J.: Compositions and methods for blackening harden steel. U.S. Patent 3,899,367, (1975)
[26] Chemical conversion coatings, black oxide coatings on iron and steel, British Standard, BS ISO 11408, (1999)
[27] R.W. Farrell: Blackening of ferrous metals, Met. Finish., 105(2007)10, 390–396, Doi: 10.1016/S0026-0576(07)80358-8
[28] R. Hurd; N. Hackerman: Kinetic studies on formation of black-oxide coatings on mild steel in alkaline nitrite solutions. J Electrochem. Soc., 104(1957)8, 482–485, Doi: 10.1149/1.2428631
[29] J. Robertson: The Mechanism of High Temperature Aqueous Corrosion of Stainless Steels. Corros. Sci. 32(1991), 443–465, Doi: 10.1016/0010-938X(91)90125-9
[30] A.R. Reghuraj; K.K. Saju: Black oxide conversion coating on metals: A review of coating techniques and adaptation for SAE 420A surgical grade stainless steel, Materials Today Proceedings, 4(2017)9, 9534–9541, Doi: 10.1016/j.matpr.2017.06.219
[31] A. Fattah-alhosseini; H.Y. Khan; A. Heidarpour: Comparison of anti-corrosive properties between hot alkaline nitrate blackening and hydrothermal blackening routes. J Alloy Compd., 676(2016) 474–480, Doi: 10.1016/j.jallcom.2016.03.114
[32] M. Eckl; S. Zaubitzer; C. Köntje; A. Farkas; L.A. Kibler; T. Jacob: An electrochemical route for hot alkaline blackening of steel: a nitrite free approach, Surfaces, 2(2019)2, 216–228, Doi:10.3390/surfaces2020017
[33] www.substech.com/dokuwiki/doku.php?id=black_copper_oxide_coating


