Micro Arc Oxidation /continued from Galvanotechnik 10/2024: Micro arc oxidation (MAO), also known as plasma electrolytic oxidation (PEO), electrolytic plasma oxidation, micro plasma oxidation, micro arc discharge oxidation, anodic spark deposition or spark anodization is a most effective and environmentally friendly way to improve the corrosion resistance, and surface mechanical properties of light metal alloys.
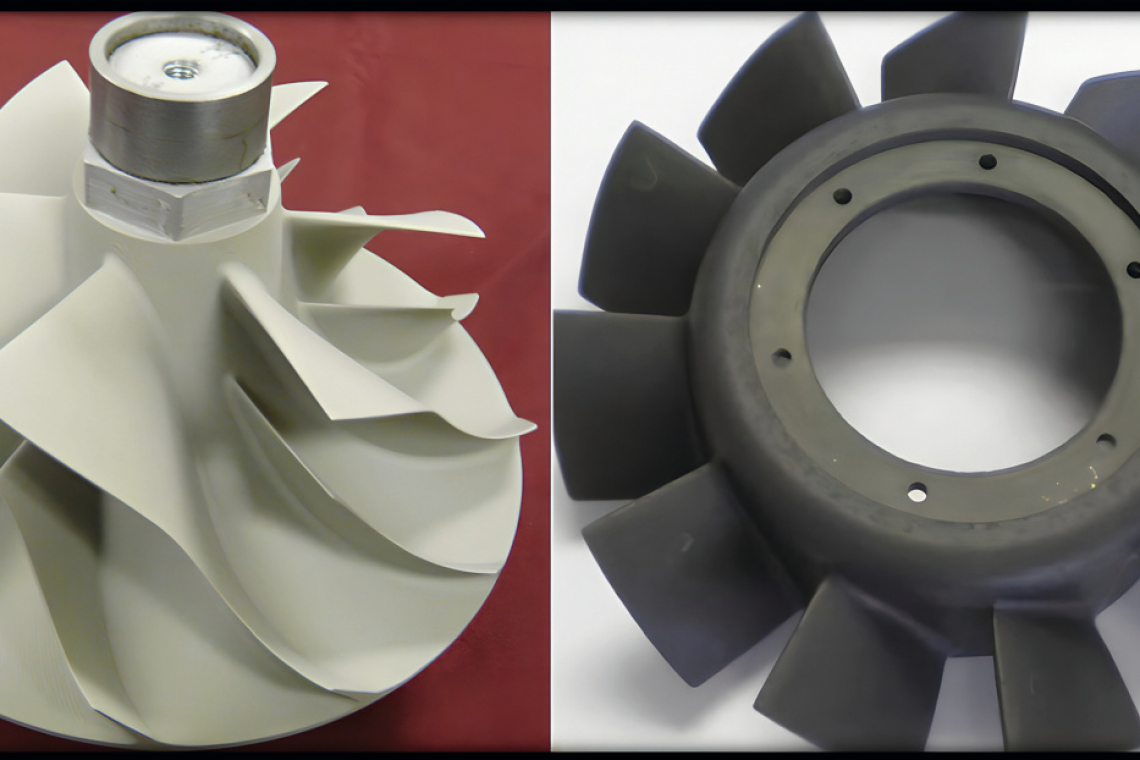
![Fig. 1: Applications of MAO coatings [7]](/images/stories/Abo-2024-11/thumbnails/thumb_GT-2024-11-064.jpg) Fig. 1: Applications of MAO coatings [7]
Fig. 1: Applications of MAO coatings [7]
The driving force for the MAO process is its simplicity. The substrate is immersed in the aqueous electrolyte and a high potential of several hundred volts is applied that trigger millions of very short-lived plasma discharges at the metal-electrolyte interface, generating high instantaneous temperatures and pressures (T > 40,000 K [> 39,727 °C] and p~100 GPa). A thick oxide film is produced on the substrate in a relatively short duration that significantly enhances its hardness, thermal resistance, dielectric strength, friction coefficient, and wear and corrosion resistance.
The Micro arc oxidation (MAO) process is similar to anodizing, but it employs sufficiently high potentials, and can be used to grow thick, largely crystalline, oxide films. The MAO layer exhibits high hardness, and excellent protection against wear, corrosion, heat or electrical insulation for various applications (Figure 1). The substrate metal gets converted into its metal oxide that grows both inwards and outwards from the original metal surface. Because it grows inward into the substrate, it has excellent adhesion. A wide range of alloys, all wrought and most cast, can be coated. The coating properties are highly dependent on the substrate, the composition of the electrolyte and the electrical regime employed. The process is rather inexpensive, and it utilizes environmentally friendly mild alkaline electrolytes. Comprehensive reviews on microarc oxidation on magnesium alloys are reported elsewhere [1-7]. A schematic of micro arc oxidation processing set-up is shown in Figure 2.
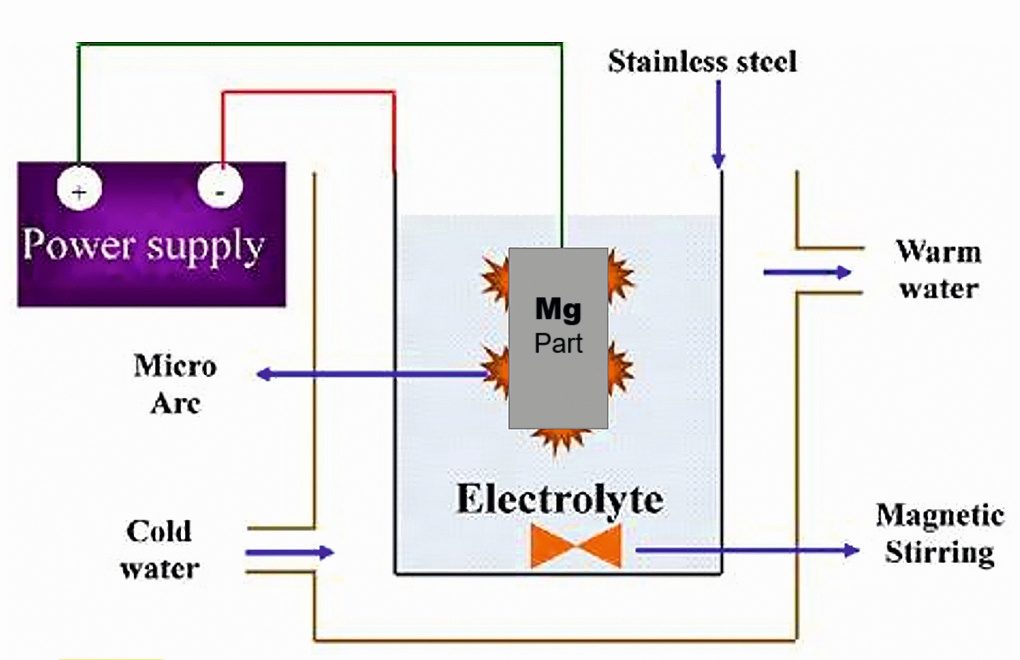 Fig. 2: Schematic of MAO process set-up
Fig. 2: Schematic of MAO process set-up
Mechanism of coating formation:
The mechanism of the PEO process can be divided into three stages, all the three stages occur simultaneously. The first stage includes oxide formation at metal-oxide interface. The second stage comprises chemical dissolution of the oxide at oxide-electrolyte interface, and the third stage involves dielectric breakdown of the oxide layer at a high voltage. The dielectric breakdown produces millions of short-lived micro-discharges uniformly spread on the surface of the job creating a discharge channel for direct ejection of molten metal which is immediately oxidized, hydrolyzed and precipitated on the workpiece. At the discharge site chemical, electrochemical, thermo-dynamical, and plasma-chemical reactions occur to modify the structure, composition and morphology of PEO coatings.
PEO coating consists of three layers: a porous top layer, a dense intermediate layer with low porosity, and an inner transition layer (Figure 3) [4]. The dense inner layer acts as a good barrier layer for corrosion resistance and determines the thermo-mechanical and tribological properties of the layer [1,4,6,8]. The coating growth rate and porosity is dependent on electrolyte components, temperature, and characteristics of applied current.
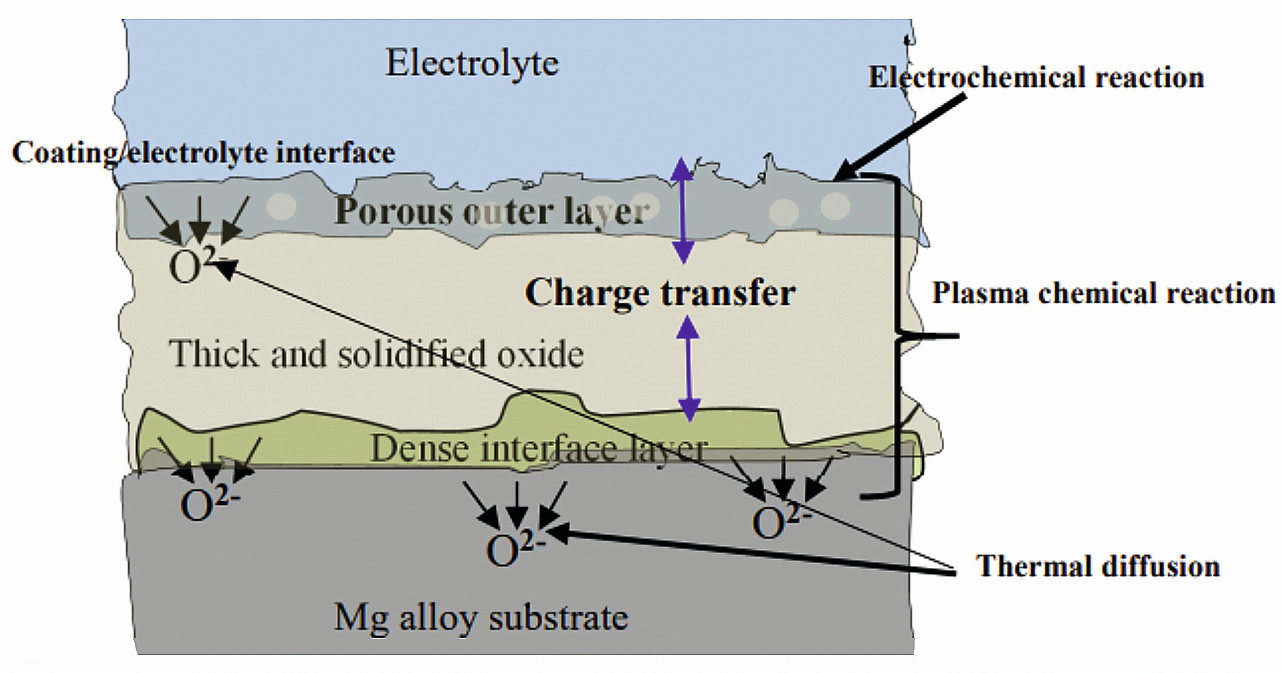 Fig. 3: Schematic of a PEO coating growth
Fig. 3: Schematic of a PEO coating growth
The mechanism of the process as explained by Yerokhin [9] is represented in Figure 4. Initially, when low anodic potentials (0 to U1) are applied to the component, the system conforms to Faraday’s laws and the current-voltage characteristics of the electrolytic cell vary according to Ohm’s law. From U1 to U2 metal passivates and an insulating barrier oxide film grows at the metal/electrolyte interface. At U2, which is the corrosion potential of the metal, the passive film begins to dissolve. When the potential is increased further (U2-U3), re-passivation occurs and a porous oxide film begins to grow. This is the regime exploited by conventional anodizing.
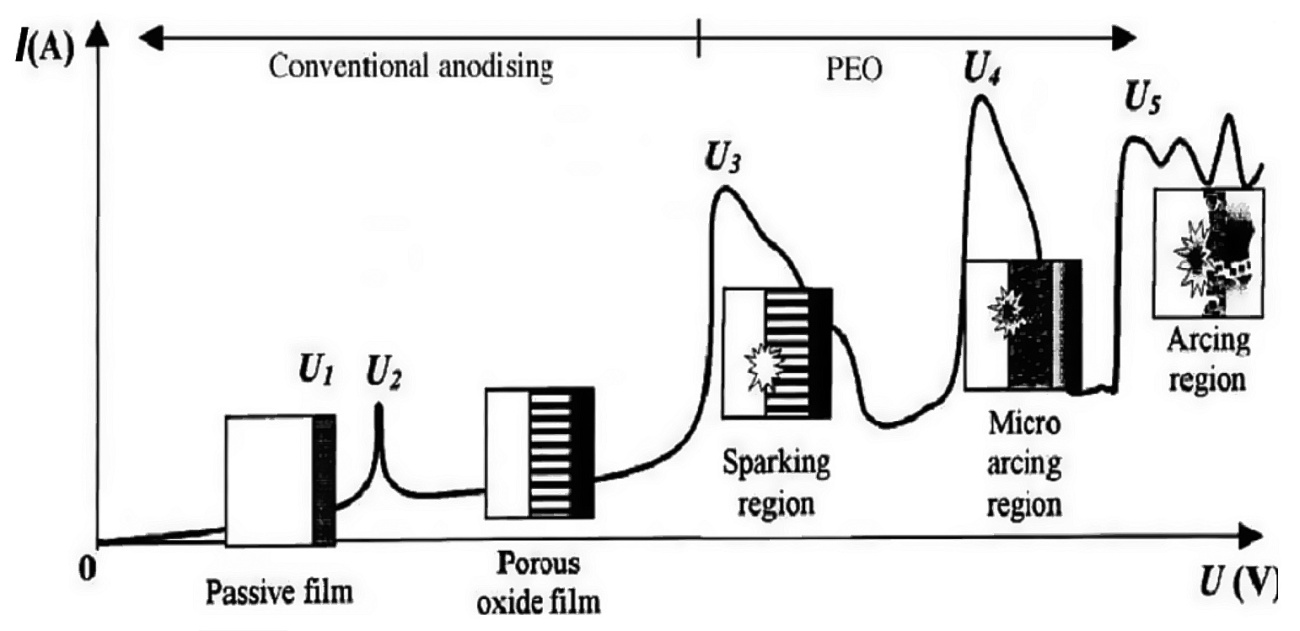 Fig. 4: Current-Voltage plot for the MAO processing regime
Fig. 4: Current-Voltage plot for the MAO processing regime
When the potential is raised further it results in dielectric breakdown of oxide film by impact ionization at discrete locations, accompanied by the visible sparks. At U4, the impact ionization is supported by the onset of thermal ionization. At U4 to U5 regime 'micro-discharges’ occur within the oxide film, the material is locally ionized, the strong electric field draws anionic components from electrolyte, and magnesium and other alloying elements from the substrate into the channel, which get oxidized to build-up the coating. In addition, consequent to the localized heating, the adjacent oxide is melted, densified and partially crystallized. Beyond U5, the micro arc discharges transform into powerful arcs, which may be destructive and inhibit further growth of the film.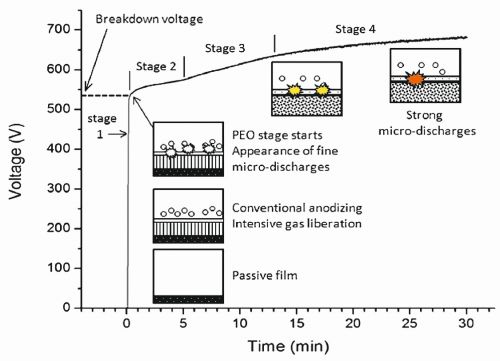 Fig. 5: Schematic of the different stages occurring during MAO process
Fig. 5: Schematic of the different stages occurring during MAO process
The subsequent arcs are more likely to occur where the oxide film is relatively thin. The discharges therefore have a natural tendency to preferentially thicken the thinnest regions of the film, resulting in fairly uniform film thickness. Schematic diagram of voltage vs. time curve under constant current mode is illustrated in Figure 5 [2].
The MAO coatings consist of porous, heterogeneous, micro cracked microstructure (Figure 6) [7,10]. The macro porosity level is generally higher of the order of 40 %, however, the bulk of the film is quite dense. The pores observed are attributed to the entrapment of spherical gas bubbles [11,12].
The most commonly used electrolytes for MAO process are alkaline solutions of phosphates, silicates, aluminates, or their combinations with some additives either to impart specific functional properties to the coating or to improve the coating growth process. The following process sequence is commonly adopted for producing MAO coating on magnesium alloys:
1. Solvent degreasing in isopropyl alcohol using an ultrasonic bath for 5-10 min. at room temperature.
2. A large number of bath formulations are investigated. Some of the typical bath formulation and operating conditions for micro arc oxidation are as follows [3,5,13,14]:
Bath formulation 1:
Potassium hydroxide, KOH 165-170 g/L
Potassium fluoride, KF 30-35 g/L
Tri sodium orthophosphate,
Na3PO4 . 12 H2O 80-95 g/L
With or without
Sodium aluminate, NaAlO2 35-90 g/L
Or
Sodium metasilicate, Na2SiO3 · 9 H2O 55-60 g/L
Bath formulation 2:
Tri sodium orthophosphate,
Na3PO4 · 12 H2O 25-35 g/L
Sodium metasilicate, Na2SiO3 · 9 H2O 10-20 g/L
Potassium fluoride, KF 1-2 g/L
Bath formulation 3:
Sodium orthosilicate, Na4SiO4 30 g/L
Sodium fluoride, NaF 10 g/L
Bath formulation 4:
Sodium fluoride, NaF: 10 g/L
Tri sodium orthophosphate,
Na3PO4 . 12 H2O 25 g/L
3. Sealing in demineralized water operating at > 95 °C (boiling) for 20-30 min.
The coating formation process can be represented by the following equations [5]:
Mg° → Mg2+ + 2 e–
Mg2+ + 2 OH− → Mg(OH)2 → MgO + H2O
2 Mg2+ + SiO32− + 2 OH− → Mg2SiO4 + H2O
Mg2+ + SiO32− → MgSiO3
Mg2+ + 2 F− → MgF2
Mg2+ + 2 AlO2− → MgAl2O4
3 Mg2+ + 2 PO43−→ Mg3(PO4)2
Magnesium dissolution reaction occurs under a strong electric field producing magnesium ions. Formation of oxide films on magnesium is due to outward diffusion of magnesium ions and inward diffusion of OH−, SiO32−, F−, AlO2− and PO43− (as the case may be depending on the electrolyte composition) under high potentials. Film formation reactions would occur once concentrations of these ions in the electrode/electrolyte interface reach a critical value. The MAO coating consists of MgO, Mg2SiO4, MgSiO3, MgF2, MgAl2O4 and Mg3(PO4)2 based on the composition of electrolyte used for electrolysis.
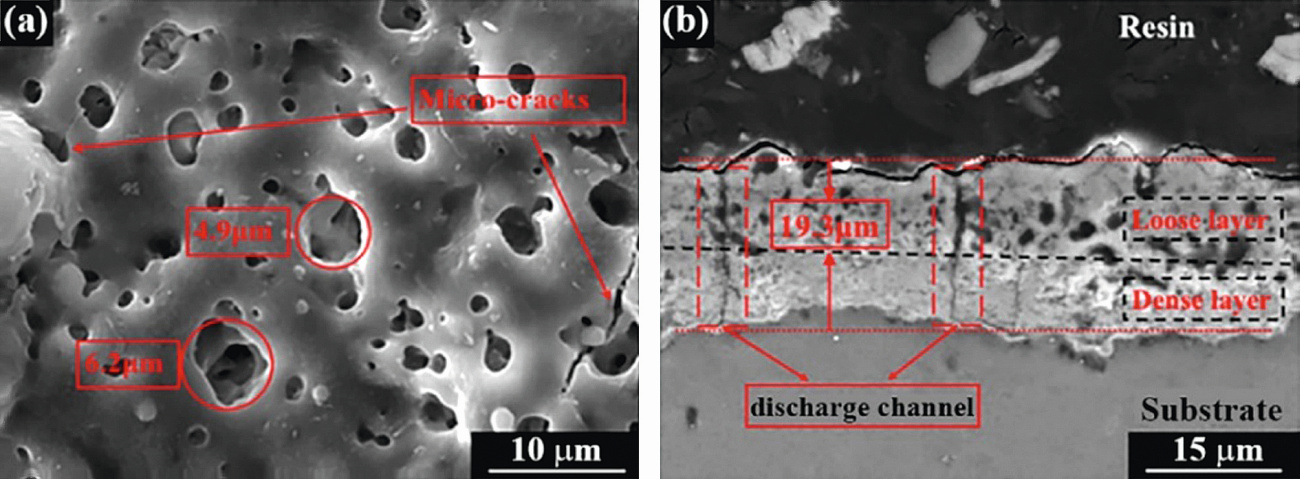 Fig. 6: (a) Surface and (b) cross-sectional morphology of MAO coatings
Fig. 6: (a) Surface and (b) cross-sectional morphology of MAO coatings
The selection of the electrolyte in particular the incorporating of oxides of Fe, Co, Ni, W, Zr, Mn, Cr and V plays an important role in realization of various colours in the MAO coatings. The coating colour changed to gray-black when V and W are incorporated into the coating from the electrolyte [15]. Black coatings have importance in thermal control applications, and to suppress stray light in the optical systems. An image of normal and black MAO coated magnesium components is shown in the picture on page 1427. Hwang et al. [16] obtained brown MAO layer on AZ91 alloy with addition of 0.07 M KMnO4 and applying a current density of 100 mA/cm2 for 300 sec. The basic electrolyte consisted of KOH (0.18 M), KF (0.09 M), and sodium silicate (0.08 M). The corrosion current density of resultant coating was dropped at least one order than that of without KMnO4.
Gun Ko and co-workers [17] formed MAO coatings with KOH: 0.5 mol/L, K4P2O7: 0.15 mol/L, NH4VO3: 0.08 mol/L; current density: 10 A/dm2; temperature: 20 °C; time: 200 sec. After 200 sec., the colour of the layer turned black due to the incorporation of vanadium oxides (V2O3 and V2O5). The corrosion resistance of the coatings was greatly improved.
A high absorptance and high emittance black MAO coating was prepared by Li et al. [18] on Mg-Li alloy with addition of vanadate in the electrolyte. The optimal conditions include 10 g/L of vanadate content and 10 min. of process time, where the coatings reached an absorptance and emittance value of 0.964 and 0.951, respectively. The coatings possessed excellent thermal stability and corrosion resistance with promising application for spacecraft. MAO coatings on AZ31 Mg alloy in an aluminate-tungstate electrolyte; NaAlO2: 10 g/L, Na2WO4 · 2 H2O: 10 g/L, C6H8O7 · H2O: 3 g/L, and KOH: 2 g/L; temperature: < 35 °C were fabricated by Tu and his team [19]. Pulsed unipolar regimes with a duty cycle of 20 % were employed with average positive and negative current densities at ~22 and ~09 A/dm2, respectively at 2000 Hz for 480 sec.
PEO based hydrophobic [20], hybrid (hydroxyapatite, double hydroxides, polymer layer) [5,7], pores filled with resins [21] etc. were investigated to enhance the surface characteristics. Dey et al. [22-24] studied the nanomechanical behaviour of MAO coatings. The values of nanohardness and Young’s modulus were ~3 and ~90 GPa, respectively. Some proprietary MAO processes are also available commercially:
Magoxide-coat treatment: This process was conceived in Russia and developed by AHC (Oberflächentechnik GmbH), Germany. It utilizes an alkaline solution consisting of phosphate, borate, silicate, aluminate or fluoride anions. The generated oxygen plasma causes partial short-term surface melting and formation of a hard oxide layer.
Keronite treatment: This process was developed in Russia and commercialized by Keronite Ltd (Cambridge, UK). Here an alternating pulsed voltage is used to produce a plasma discharge on the surface of light metals (Al, Mg, Ti) in an aqueous solution.
Operating Conditions for MAO process:
Temperature 25 ± 5 °C
Current density 2.0 ± 0.5 A/dm2
Voltage 0 to 500 V
Agitation moderate
Tank material PVC or PP with
Immersion heaters
Time 15-20 min.
Coating thickness 20 ± 5 µm
REFERENCES:
[1] F.C. Walsh; C.T.J. Low; R.J.K. Wood; K.T. Stevens; J. Archer; A.R. Poeton; A. Ryder: Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys, Trans. Inst. Met. Finish., 87, no. 3 (2009) 122-135. doi: 10.1179/174591908X372482
[2] B.L. Jiang; Y.F. Ge: Micro-arc oxidation (MAO) to improve the corrosion resistance of magnesium (Mg) alloys (Chapter 7), Corrosion Prevention of Magnesium Alloys, G.-L. Song (Editor), Woodhead Publishing, (2013) 163-196. doi: 10.1533/9780857098962.2.163
[3] B.V. Vladimirov; B.L. Krit; V.B. Lyudin; N.V. Morozova; A.D. Rossiiskaya; I. V. Suminov; A. V. Epel’feld: Microarc discharge oxidizing of magnesium alloys: A review, Surf. Eng. Appl. Electrochem., 50 (2014) 195-232. doi: 10.3103/S1068375514030090
[4] R.O. Hussein; D.O. Northwood: Production of Anti-Corrosion Coatings on Light Alloys (Al, Mg, Ti) by Plasma-Electrolytic Oxidation (PEO) (Chapter 11), Developments in Corrosion Protection, M. Aliofkhazraei (Editor), IntechOpen, (2014) 201-239. doi: 10.5772/57171
[5] G.B. Darband; M. Aliofkhazraei; P. Hamghalam; N. Valizade: Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications, J. Magnes. Alloy., 5, no. 1 (2017) 74-132. doi: 10.1016/j.jma.2017.02.004
[6] Q. Li; J. Liang; Q. Wang: Plasma Electrolytic Oxidation Coatings on Lightweight Metals (Chapter 4), Modern Surface Engineering Treatments, M. Aliofkhazraei (Editor), IntechOpen, (2013) 75-99. doi: 10.5772/55688
[7] W. Yao; L. Wu, J. Wang, B. Jiang, D. Zhang, M. Serdechnova, T. Shulha, C. Blawert, M.L. Zheludkevich, F. Pan: Micro-arc oxidation of magnesium alloys: A review, J. Mater. Sci. Technol., 118 (2022) 158-180. doi: 10.1016/j.jmst.2021.11.053
[8] G. Wirtz; S. Brown; W. Kriven: Ceramic coatings by anodic spark deposition, Mater. Manuf. Process., 6 (1991) 87-115. doi: 10.1080/10426919108934737
[9] A.L. Yerokhin; X. Nie; A. Leyland; A. Matthews; S.J. Dowey: Plasma electrolysis for surface engineering, Surf. Coat. Technol., 122, no. 2-3 (1999) 73-93. doi: 10.1016/S0257-8972(99)00441-7
[10] Z. Chen; C. Bao; Y. Jian; Q. Wang; F. Chen: Microstructure and corrosion resistance of SiC nanoparticles reinforced ceramic composite coating on Mg-Li based composite by micro-arc oxidation, J. Electrochem. Soc., 166 (2019) C571-C579. doi: 10.1149/2.1181915jes
[11] F.A. Bonilla; E. Berkani; Y. Liu; P. Skeldon; G.E. Thompson; H. Habazaki; K. Shimizu; C. John; K. Stevens: Formation of anodic films on magnesium alloys in an alkaline phosphate electrolyte, J. Electrochem. Soc., 149, no. 1 (2002) B4-B13. doi: 10.1149/1.1424896
[12] S. Durdu; A. Aytac; M. Usta: Characterization and corrosion behavior of ceramic coating on magnesium by micro-arc oxidation, J. Alloys Compd., 509, no. 34 (2011) 8601-8606. doi: 10.1016/j.jallcom.2011.06.059
[13] X.M. Song; G. Yu; H.B. Yi; L.Y. Yel; B.N. Hu: Phosphate-silicate composite coating formed on AM60 magnesium alloy, Surf. Eng., 26, no. 5 (2010) 371-377. doi: 10.1179/026708409X12450792800114
[14] O. Khaselev; D. Weiss; J. Yahalom: Anodizing of pure magnesium in KOH-aluminate solutions under sparking, J. Electrochem. Soc., 146, no. 5 (1999) 1757-1761. doi: 10.1149/1.1391838
[15] A.K. Sharma: Black electrochemical coatings for aerospace and allied applications, Part 7- Black Plasma Electrolytic Oxidation (PEO) coatings, Galvanotechnik, 112, no. 12 (2021) 1587-1592
[16] D.Y. Hwang; Y.M. Kim; D.Y. Park; B. Yoo; D.H. Shin: Corrosion resistance of oxide layers formed on AZ91 Mg alloy in KMnO4 electrolyte by plasma electrolytic oxidation, Electrochim. Acta, 54, no. 23 (2009) 5479-¬5485. doi: 10.1016/j.electacta.2009.04.047
[17] Y. Gun Ko; K.M. Lee; D. Hyuk Shin: Effect of ammonium metavanadate on surface characteristics of oxide layer formed on Mg alloy via plasma electrolytic oxidation, Surf. Coat. Technol., 236 (2013) 70-74. doi: 10.1016/j.surfcoat.2013.08.060
[18] X. Li; Q. Xia; C. Chen; Z. Yao: Preparation of high absorptance and high emissivity coatings on Mg-Li alloy by plasma electrolytic oxidation, Meter. Res. Exp., 6, no. 10 (2019) 106428. doi: 10.1088/2053-1591/ab3d8d
[19] W. Tu; W. Zhu; X. Zhuang; Y. Cheng; P. Skeldon: Effect of frequency on black coating formation on AZ31 magnesium alloy by plasma electrolytic oxidation in aluminatetungstate electrolyte, Surf. Coat. Technol., 372 (2019) 34-44. doi: 10.1016/j.surfcoat.2019.05.012
[20] S.S. Farhadi; M. Aliofkhazraei; G. B. Darband; A. Abolhasani; A. S. Rouhaghdam: Corrosion and wettability of PEO coatings on magnesium by addition of potassium stearate, J. Magnes. Alloy., vol. 5, 2017, 210-216. doi: 10.1016/j.jma.2017.06.002
[21] Y. Ge; B. Jiang; M. Liu; C. Wang; W. Shen: Preparation and characterization of the micro-arc oxidation composite coatings on magnesium alloys, J. Magnes. Alloy., vol. 2, 2014, 309-316. doi: 10.1016/j.jma.2014.11.006
[22] A. Dey; R. Uma Rani; H.K. Thota; A.K. Sharma; P. Bandyopadhyay; A.K. Mukhopadhyay: Microstructural, corrosion and nanomechanical behaviour of ceramic coatings developed on magnesium AZ31 alloy by micro arc oxidation, Ceram. Int, 39, no. 3 (2013) 3313-3320. doi: 10.1016/j.ceramint.2012.10.020
[23] A. Dey; R. Uma Rani; H.K. Thota; A. Rajendra; A.K. Sharma; P. Bandyopadhyay; A.K. Mukhopadhyay: Nanoindentation study of MAO coatings developed by dual electrolytes, Surf. Eng., 30, no. 12 (2014) 905-912. doi: 10.1179/1743294413Y.0000000239
[24] A. Dey; R. Uma Rani; H.K. Thota; P. Bandyopadhyay; A. Rajendra; A.K. Sharma; A.K. Mukhopadhyay: Corrosion and nanoindentation studies of MAO coatings, Surf. Eng., 30, no. 12 (2014) 913-919. doi: 10.1179/1743294413Y.0000000244