– Part 1 – General Introduction
Corrosion has a significant impact on active metals such as iron, magnesium, aluminium, copper, and their alloys. It can compromise the structural integrity of buildings, bridges, marine vessels, airplanes, tunnels, and pipelines, posing substantial safety risks to individuals and the environment. Electronic components and circuitry may also suffer from corrosion, leading to malfunctions, diminished performance, and potential failure. This article series provides an overview of different types of corrosion processes, their basic mechanisms, strategies for mitigation, and techniques for measuring corrosion rates.
Corrosion is defined as the degradation of a metal due to chemical, electrochemical, and biochemical interaction with its environment. It is the reverse process of metallurgy, where refined metals gradually deteriorate and transform into a more chemically stable form such as oxide, hydroxide, carbonates or sulphide by reactions with their surroundings. Only precious metals are exceptions, as they occur naturally in their pure metallic state. All other metals are derived from minerals or ores which are inherently unstable in their natural environments [1-6]. Metals positioned higher in the reactivity series, such as magnesium, zinc, aluminium, and iron, are prone to corrosion, whereas those lower in the series, like platinum, and gold, have very high resistance to corrosion. This is attributed to the decreasing tendency to undergo oxidation as one moves down the series, which is evident from their lower oxidation potentials.
Corrosion diminishes the beneficial properties of materials and structures, such as mechanical strength, appearance, and resistance to liquids and gases. Corrosion fatigue refers to the premature fracturing of metal caused by the combined effects of corrosion and cyclic loading, occurring at lower stress levels or after fewer cycles than in a non-corrosive environment. Corrosion fatigue strength is defined as the maximum repeated stress a metal can endure without failure under specific conditions of corrosion and cyclic loading, over a specific number of stress cycles and time duration [4]. While corrosion can affect non-metallic materials like ceramics and polymers, the term “degradation“ is more commonly used to describe such deterioration of materials rather than corrosion. Corrosion holds significant economic importance as all actions undertaken to prevent or manage it, as well as its outcomes, involve supplementary expenses.
Corrosion can impact various metals differently, and the same metal can suffer varying degrees of corrosion depending on the environment. It primarily leads to the wastage of natural resources. Moreover, it can create dangerous situations, including weakened and unstable building structures, accidents due to corroded components, and other failures like pipeline ruptures, bridge collapses, and transportation breakdowns. Based on the study of National Association of Corrosion Engineers (NACE) International in 2016, the cost of corrosion is estimated more than 2.5 trillion US dollars (2,15 trillion euros) globally each year, which amounts to 3.4 % of the Gross Domestic Product (GDP), due to repairs, maintenance, and the replacement of damaged equipment and infrastructure [7,8]. Comprehending and combating corrosion is vital for safeguarding infrastructure, ensuring safety, and reducing economic losses.
Factors Affecting Corrosion
Many materials corrode merely by being exposed to moisture in the air; however, this process can be significantly accelerated by certain corrosive environmental factors:
- Exposure to saltwater or air containing compounds such as CO2, SO2, and SO3 can significantly accelerate the rate of corrosion.
- An increase in temperature can hasten the corrosion process.
- The nature of the oxide layer that forms on the surface of metal is crucial. Metals like aluminium form an insoluble layer of Al2O3, which provides moderate protection against further corrosion. In contrast, metals such as iron develop rust that can crumble and expose more metal to corrosion.
- The presence of acid mists in the atmosphere can greatly speed up corrosion rate.
Prevention of Corrosion
Preventing corrosion is essential to avoid substantial losses. The primary strategy for mitigating corrosion is to apply a protective coating on the surface of metal that blocks water, oxygen, and other corrosive agents. Popular methods include electroplating, galvanization, anodization, passivation, biofilm coatings, painting, oiling, and greasing. However, the protective coating must be intact; incomplete coverage can sometimes hasten corrosion by restricting oxygen access to certain areas of the surface. An illustrative example of this is when iron is coated with a less reactive metal like tin. Even a tiny hole in the tin can lead to rapid localized rusting. The tin, with a higher electrode potential, becomes cathodic and protects itself, while the exposed iron becomes anodic and undergoes rapid oxidation by oxygen.
A second technique involves placing the metal object that requires protection in contact with a more active metal. This method is known as cathodic protection, where the more reactive metal provides electrons, thereby greatly reducing corrosion. Galvanizing provides both surface coating and cathodic protection to iron objects by plating zinc onto steel, either electrolytically or through immersion in molten metal. Zinc serves as a self-protective layer, reacting with oxygen and carbon dioxide in the air to form a dense, protective coating of zinc hydroxycarbonate, Zn2(OH)2CO3. If the zinc coating is scratched, the underlying iron remains protected from rusting as zinc is preferentially oxidized. The resulting hydroxycarbonate seals the scratch, blocking further exposure of the iron or zinc to oxygen.
The third technique involves the application of corrosion inhibitors. This is relevant in certain scenarios, such as an automobile radiator, where aqueous solutions come into contact with iron. These inhibitors encompass chromate salts and organic compounds such as tributylamine, (C4H9)3N. Chromates form a protective coating of FeCrO4 as soon as any iron is oxidized to iron(II). Tributylamine, an ammonia derivative, reacts with organic acids produced from the decomposition of antifreeze at high engine temperatures. The resulting tributylammonium salts are insoluble, creating a protective layer inside the cooling system. Consequently, tributylamine neutralizes acids that would otherwise hasten corrosion, and also provides a protective surface coating. A range of corrosion inhibitors is tailored for particular metals and corrosive environments. This outlines general strategies for corrosion protection. There are various types of corrosion, each affected by the specific metals involved and the environmental conditions. Specific corrosion prevention methods suitable for each type of corrosion will be addressed in subsequent discussions.
Typical Examples of Corrosion
In this discussion, we will explore some common examples of general corrosion that are frequently encountered in our daily lives.
Rusting of Iron
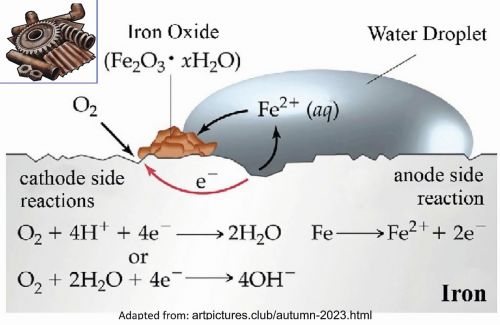
Rusting of iron is commonly seen when it comes in contact with air or water. This can be explained by a typical electrochemical cell reaction as depicted in Figure 1.
Here, iron loses electrons and gets converted to Fe(aq)2+ (this could be considered as the anode position). The electrons lost will move to the other side, where they combine with H+ ions. H+ ions are released either by H2O or by H2CO3 present in the atmosphere (this could be considered as the cathode position).
H2O ⇌ H+ + OH−
H2CO3 ⇌ 2 H+ + CO32−
The Hydrogen, thus formed by the reaction of H+ and electrons, react with oxygen to form H2O.
Anodic reaction: 2 Fe → 2 Fe2+ + 4 e–
Cathodic reaction: O2 + 4 H+ + 4 e− → 2 H2O
Overall reaction: 2 Fe + O2 + 4 H+ → 2 Fe2+ + 2 H2O
The Fe2+ ions formed at the anode react with oxygen in the atmosphere, and thereby get oxidised to Fe3+ and forming hydrated Fe2O3 (rust).
4 Fe2+ + 3 O2 → 2 Fe2O3
Fe2O3 + x H2O → Fe2O3.x H2O (rust)
Iron and its alloys undergo different forms of corrosion depending on the environment to which they are exposed. These forms are discussed in the subsequent parts.
Magnesium Corrosion
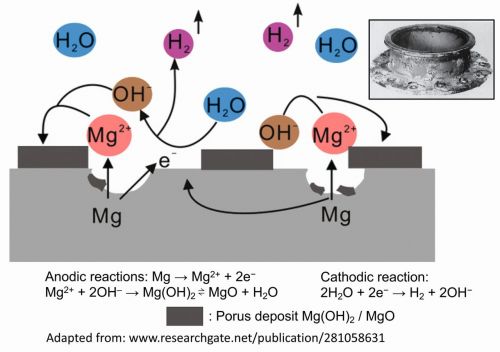
Magnesium is susceptible to rapid corrosion due to its high reactivity. It reacts severely with air and water, resulting in the formation of an instant oxide-carbonate film on the surface. This film is porous and, unlike aluminium oxide films on aluminium, is not self-healing; consequently, it does not offer protection. The high reactivity of magnesium is evident from its position in the electromotive force, or galvanic series, where it has a standard electrode potential (E°) of -2.37 V. Figure 2 demonstrates the corrosion process of magnesium in an aqueous medium [9,10].
The anodic and cathodic reactions in the corrosion process can be represented as follows:
Anodic reactions:
Mg → Mg2+ + 2 e−
Mg2+ + 2 OH– → Mg(OH)2 ⇌ MgO + H2O
Side reaction in presence of CO2:
Mg(OH)2 + 2 CO2 → Mg(HCO3)2
Mg2+ + 2 HCO3− → MgCO3 + CO2 + H2O
Cathodic reaction:
2 H2O + 2 e− → H2 + 2 OH−
Magnesium alloys undergo complex transitional changes during the corrosion process. The primary types of corrosion include general, localized, galvanic, and stress corrosion cracking. Galvanic corrosion is the typical form of magnesium alloy deterioration, while stress corrosion cracking represents a combined failure mode of both corrosion and environmental factors [11].
Aluminium Corrosion
Aluminium, being highly reactive, instantly forms a tenacious film of aluminium oxide when exposed to air or other oxygen-rich environments. This thin, natural, amorphous layer of aluminium oxide on the surface offers some protection to aluminium in mildly corrosive settings. Although the film is an electrical insulator, it conducts heat relatively well, with a thermal conductivity of 30 Wm–1K–1. Oxygen plays a significant role in the corrosion of aluminium. The rate of attack escalates with higher concentrations of dissolved oxygen, particularly in acidic solutions. Conversely, in deaerated solutions, the corrosion rate is markedly slow. Schematic depiction of aluminium corrosion in water is shown in Figure 3.
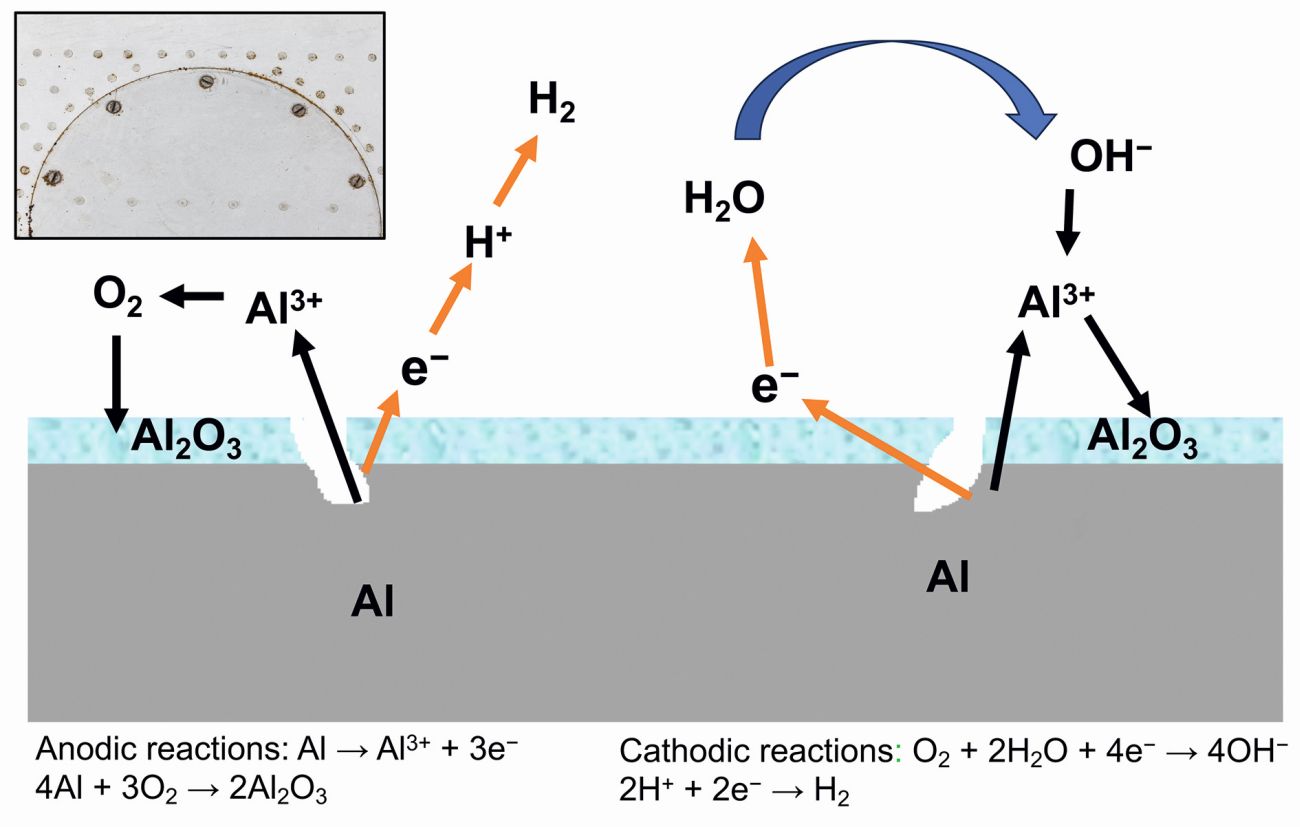 Fig. 3: Depiction of aluminium corrosion in water
Fig. 3: Depiction of aluminium corrosion in water
Aluminium can react with water in the following ways:
2 Al + 6 H2O → 2 Al(OH)3 + 3 H2
2 Al + 4 H2O → 2 AlO(OH) + 3 H2
2 Al + 3 H2O → Al2O3 + 3 H2
The general atmospheric corrosion reactions of aluminium in water / air can be shown by the following equations [12,13]:
Anodic reactions:
Al → Al3+ + 3 e−
4 Al + 3 O2 → 2 Al2O3
2 Al3+ + 6 (OH)− → 2 Al(OH)3 ⇌ Al2O3 + 3 H2
Al2O3 + H2O → Al2O3.H2O
Cathodic reactions:
O2 + 2 H2O + 4 e− → 4 OH−
2 H+ + 2 e− → H2
These reactions are thermodynamically favourable from room temperature up to aluminium’s melting point at 660 °C and are highly exothermic. Al(OH)3 is the most stable product between room temperature and 280 °C. From 280 °C to 480 °C, AlO(OH) has greater stability. Above 480 °C, the most stable form is Al2O3.
Copper corrosion
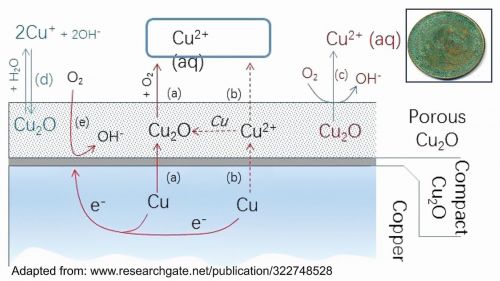
 Fig. 4: Colour of Statue of Liberty in 1900’s and 2020’sWhen copper is exposed to the environment, it reacts with the atmospheric oxygen to form copper (I) oxide, which is red in colour. Cu2O gets further oxidised to CuO, which is black in colour. The thin, compact inner layer, typically formed at the metal-atmosphere interface, serves to protect the metal from further corrosion to a certain degree, whereas the more porous outer layer continues to react with the atmosphere.
Fig. 4: Colour of Statue of Liberty in 1900’s and 2020’sWhen copper is exposed to the environment, it reacts with the atmospheric oxygen to form copper (I) oxide, which is red in colour. Cu2O gets further oxidised to CuO, which is black in colour. The thin, compact inner layer, typically formed at the metal-atmosphere interface, serves to protect the metal from further corrosion to a certain degree, whereas the more porous outer layer continues to react with the atmosphere.
2 Cu + ½ O2 → Cu2O [Red]
Cu2O + ½ O2 → 2 CuO [Black]
CuO reacts with atmospheric CO2, SO3 and H2O present in the atmosphere to form Cu2(OH)2CO3 (Malachite), which is bluish green, and Cu4SO4(OH)6 (Brochantite), which is green in colour. This reaction is the reason copper exhibits a bluish-green colour over time. A notable illustration of this phenomenon is the Statue of Liberty (Fig. 4), where the copper coating has turned blue-green. Figure 5 illustrates the corrosion process of copper schematically [14].
Silver Tarnishing
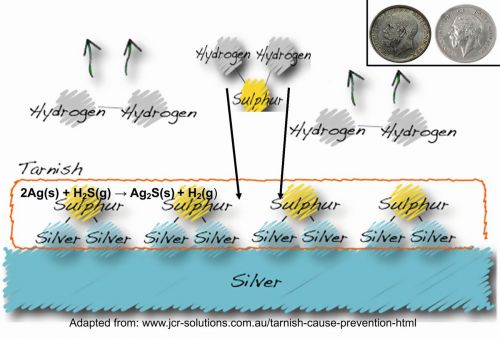
Silver does not react with oxygen in the air at room temperature. However, silver objects gradually tarnish and turn black over time when exposed to moist air. This is due to the presence of trace amounts of sulphur compounds (such as H2S) in the atmosphere, which react with silver to form silver sulphide (Ag2S), resulting in a dark brown to black coloration.
2 Ag + H2S → Ag2S + H2
2 Ag + H2S + ½ O2 → Ag2S + H2O
Some regions naturally exhibit elevated sulphur levels, including areas with dense traffic contributing to higher exhaust emissions, zones with substantial industrial activity, volcanic areas, and hot springs, among others. Figure 6 depicts the process of silver tarnishing in presence of sulphide.
In dieser 11-teiligen Serie widmen wir uns jedem Detail des Megathemas Korrosion - von allen Arten der Korrosion, inklusive Hochtemperaturkorrosion und mikrobiologisch beeinflusster Korrosion, über das Pourbaix-Diagramm bis zur Bestimmung der Korrosionsrate.
REFERENCES:
[1] K.A. Natarajan: Principles of Corrosion Processes. In Corrosion Processes. Structural Integrity, G. Vachtsevanos; K. Natarajan; R. Rajamani; P. Sandborn (Editors), Springer, Cham., vol. 13, (2020) 27-82. doi: 10.1007/978-3-030-32831-3_2
[2] B.N. Popov: Corrosion Engineering. In Principles and Solved Problems, Elsevier, 2015, 1-758. doi: 10.1016/C2012-0-03070-0
[3] C.M. Hussain; C. Verma; J. Aslam; R. Aslam; S. Zehra: Handbook of Corrosion Engineering: Modern Theory, Fundamentals and Practical Applications, Elsevier, 2023, 1-432. doi: 10.1016/C2021-0-02205-7
[4] P.R. Roberge: Handbook of Corrosion Engineering, McGraw-Hill LLC, First Edition, 1999, 1-1128; Second Edition, 2012, 1-994; Third Edition, 2019, 1-790. https://dl.icdst.org/pdfs/files/441d337b7410198db6d96e61a6716302.pdf
[5] B.A. Omran; M.O. Abdel-Salam: Basic Corrosion Fundamentals, Aspects and Currently Applied Strategies for Corrosion Mitigation. In A New Era for Microbial Corrosion Mitigation Using Nanotechnology, Adv. Mat. Res., Springer, Cham., 2020, 1-45. doi: 10.1007/978-3-030-49532-9_1
[6] Corrosion Fundamentals: NASA. https://public.ksc.nasa.gov/corrosion/corrosion-fundamentals. Accessed on April 14, 2025
[7] M. Yeganeh; T.A. Nguyen; S. Rajendran; S. Kakooei; Y. Li: Chapter 1: Corrosion Protection at the Nanoscale: An Introduction. In Micro and Nano Technologies, S. Rajendran; T.A. Nguyen; S. Kakooei; M. Yeganeh; Y. Li (Editors), Elsevier, 2020, 3-7. doi: 10.1016/B978-0-12-819359-4.00001-5
[8] M.A.J. Mazumder: Global impact of corrosion: Occurrence, cost and mitigation, Global J. Eng. Sci., 5, no. 4 (2020) 000618. doi: 10.33552/GJES.2020.05.000618. https://irispublishers.com/gjes/fulltext/global-impact-of-corrosion-occurrence-cost-and-mitigation.ID.000618.php
[9] R. Zhang; X.T. Li; S.Q. Li; F. Zhang: In vitro degradation of pure Mg in response to glucose, Sci. Rep., 5, no. 1 (2015) 13026. doi:10.1038/srep13026
[10] A.I. Bita; I. Antoniac; I. Ciuca: Potential use of Mg-Ca alloys for orthopedic applications, U.P.B. Sci. Bull., Series B: Chem. Mater. Sci., 78, no. 3 (2016) 173-184. https://www.scientificbulletin.upb.ro/rev_docs_arhiva/fullc85_961002.pdf
[11] T. Wu; K. Zhang: Corrosion and protection of magnesium alloys: Recent advances and future perspectives, Coatings, 13, no. 9 (2023) 1533. doi: 10.3390/coatings13091533. https://www.mdpi.com/2079-6412/13/9/1533
[12] Aluminium Information Portal, Aluminium Guide, Chemical reactions of aluminium, https://aluminium-guide.com/chemical-reactions-aluminium. Accessed on April 14, 2025
[13] Francisco Servigna: The Chemical Nature of Aluminum and Aluminum Corrosion. https://www.corrosionpedia.com/understanding-aluminum-corrosion/2/6954
[14] P. Zhou: An in situ kinetic investigation of the selective dissolution mechanism of Cu alloys, Ph.D. Thesis, Pierre and Marie Curie University, 2017, 1-181. (p. 28). https://www.researchgate.net/publication/322748528