PFAS: The Persistent Threat of Forever Chemicals
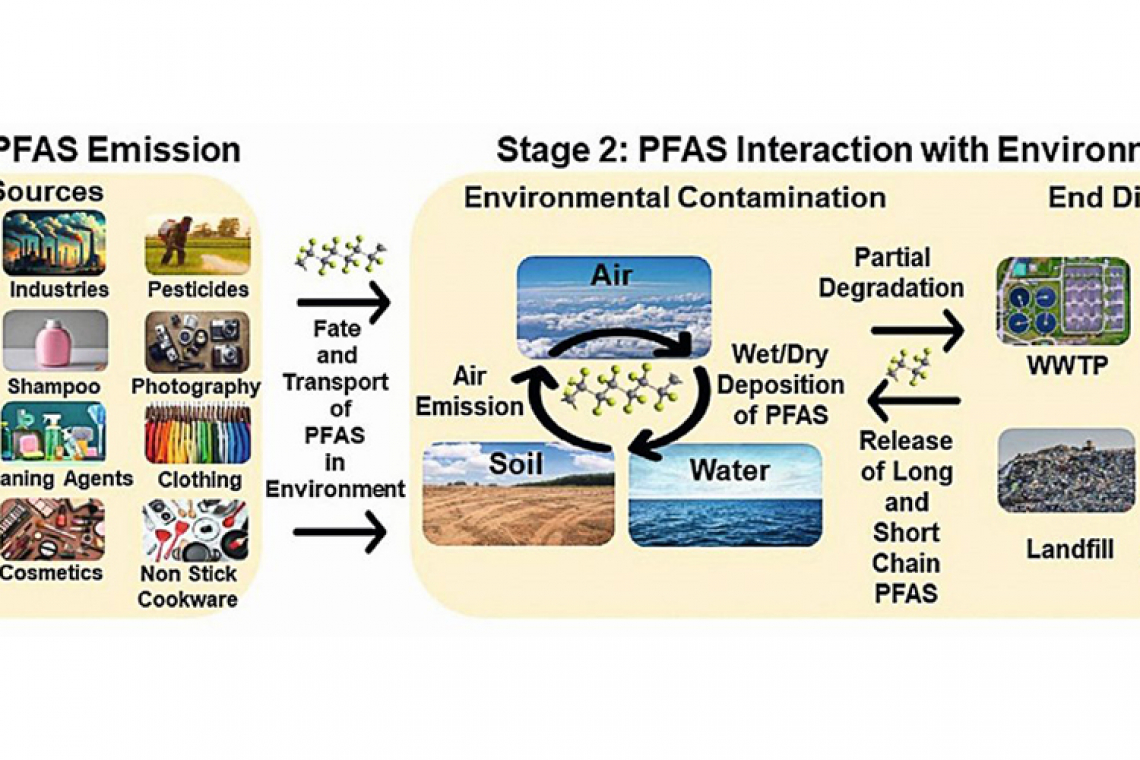
Per- and polyfluoroalkyl substances (PFAS), known as “forever chemicals”, are synthetic compounds used in products like nonstick cookware, food packaging, firefighting foams, and cosmetics due to their water-, grease-, and heat-resistant properties. Their strong carbon-fluorine bonds make them highly persistent in the environment, accumulating in soil, water, and air. Figure 1 [1] illustrates PFAS emission and migration pathways, including deposition and entry into the food chain via landfills, wastewater, drinking water treatment plants (WWTP, DWTP), and human consumption. Significant research has been conducted on PFAS in recent years [1-10].
Environmental and Health Risks of PFAS
PFAS contamination has been found in soil, groundwater, surface water, and air. Their persistence allows them to bioaccumulate in living organisms, posing severe health risks, as shown in Figure 2 [4].
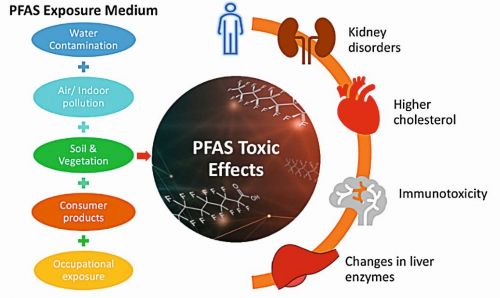 Fig. 2: Major health effects of PFAS
Fig. 2: Major health effects of PFAS
- Immune system suppression – Reduced vaccine effectiveness and increased infection risk.
- Increased cancer risk – Long-term exposure linked to kidney, testicular, and other cancers.
- Hormonal and metabolic disruption – Interferes with endocrine function, causing thyroid disorders, developmental delays, and reproductive issues.
- Liver and kidney damage – Prolonged exposure leads to organ toxicity and related complications.
- High cholesterol and blood pressure - Increases the risk of cardiovascular diseases.
Challenges in PFAS Removal
PFAS are difficult to manage due to their chemical stability, mobility, and resistance to conventional water treatment methods. Standard processes like coagulation, sedimentation, filtration, and biological degradation are largely ineffective. Technologies such as activated carbon and ion-exchange resins can capture PFAS but do not destroy them, raising concerns about secondary waste disposal. Chemical treatments may also produce harmful byproducts. Emerging solutions, particularly electrochemical treatments, offer more effective PFAS degradation with higher defluorination rates.
Electrochemical Methods for PFAS Treatment
Electrochemical treatment processes use electrical energy to degrade or remove PFAS from water. Key techniques include Electrochemical Oxidation (EO), Electrocoagulation (EC), and Electrosorption. EO breaks down PFAS molecules through direct and advanced oxidation reactions, EC removes PFAS through coagulation-precipitation followed by separation, and Electrosorption captures PFAS via electrostatic attraction and adsorption.
Electrochemical Oxidation (EO)
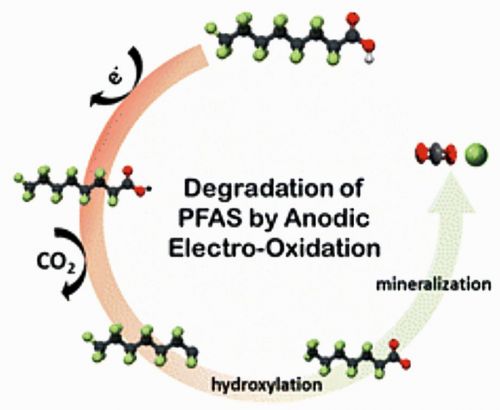
EO is an advanced wastewater treatment method that uses anodic oxidation instead of chemical oxidation to degrade contaminants. It generates reactive species through oxidative reactions at the anode and reductive reactions at the cathode, effectively mineralizing both biodegradable and non-biodegradable organic pollut-ants. In PFAS remediation, EO is particularly effective due to its ability to break C–F bonds, converting PFAS into harmless byproducts like fluoride and carbon dioxide. High-performance anodes such as boron-doped diamond (BDD) and Magnéli phase titanium suboxides enhance degradation efficiency. Unlike conventional treatment methods that merely capture PFAS, EO provides a complete degradation pathway, minimizing the risk of secondary contamination (Fig. 3) [5]. EO can also be integrated with adsorption or membrane filtration to improve overall treatment efficiency, making it a leading solution in PFAS remediation research.
Electrocoagulation (EC)
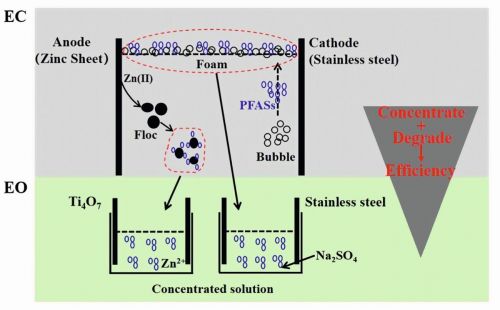 Fig. 4: Electrocoagulation and electrochemical oxidationEC uses an electric current to destabilize and remove contaminants without chemical coagulants. It generates metal ions in situ from sacrificial aluminium or iron electrodes, facilitating pollutant removal through charge neutralization, precipitation, and adsorption. In PFAS remediation, these flocs capture PFAS, which are then removed through sedimentation or filtration. Electrostatic interactions and electro-flocculation further improve separation efficiency. It offers advantages such as minimal chemical use, cost-effectiveness, and the ability to treat large water volumes. EC can also be integrated with EO for enhanced degradation of PFAS (Fig. 4) [9]. Current research aims to optimize parameters—like electrode material (e.g., zinc anodes), current density, and operating conditions—for improved efficiency and scalability.
Fig. 4: Electrocoagulation and electrochemical oxidationEC uses an electric current to destabilize and remove contaminants without chemical coagulants. It generates metal ions in situ from sacrificial aluminium or iron electrodes, facilitating pollutant removal through charge neutralization, precipitation, and adsorption. In PFAS remediation, these flocs capture PFAS, which are then removed through sedimentation or filtration. Electrostatic interactions and electro-flocculation further improve separation efficiency. It offers advantages such as minimal chemical use, cost-effectiveness, and the ability to treat large water volumes. EC can also be integrated with EO for enhanced degradation of PFAS (Fig. 4) [9]. Current research aims to optimize parameters—like electrode material (e.g., zinc anodes), current density, and operating conditions—for improved efficiency and scalability.
Electrosorption
Electrosorption is an emerging electrochemical technique that uses an electric field to capture and remove PFAS in contaminated water by adsorbing them onto charged electrode surfaces. Unlike conventional adsorption, which relies solely on porous materials like activated carbon or ion-exchange resins, electrosorption enhances removal efficiency and selectivity by applying an external voltage.
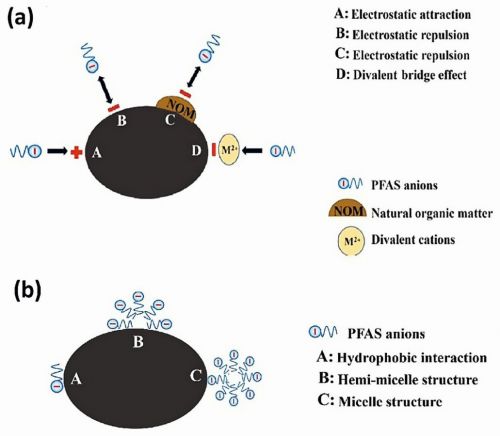 Fig. 5: (a) Divalent bridge effect, electrostatic attraction, and repulsion in PFAS adsorption, (b) Adsorption of PFAS through hydrophobic interactions
Fig. 5: (a) Divalent bridge effect, electrostatic attraction, and repulsion in PFAS adsorption, (b) Adsorption of PFAS through hydrophobic interactions
|
Parameter |
Electro-oxidation (EO) |
Electrocoagulation (EC) |
Electrosorption |
|
Principle |
Oxidation of PFAS at the anode, breaking C-F bonds |
In-situ generation of metal ions that adsorb and precipitate PFAS |
Electrostatic adsorption of PFAS onto charged electrode surfaces |
|
PFAS Removal Mechanism |
Complete mineralization into CO2 and fluoride ions |
PFAS capture via coagulation and precipitation |
PFAS adsorption onto electrode surfaces |
|
Efficiency |
High degradation and defluorination rates |
Effective in removing PFAS but does not degrade them |
Efficient at PFAS removal but does not break C-F bonds |
|
Secondary Waste |
Minimal, as PFAS are destroyed |
Moderate, due to sludge formation |
Minimal, with potential for electrode regeneration |
|
Energy Requirement |
High, due to the need for advanced anodes and oxidation reactions |
Moderate, depends on electrode material and current density |
Low, as adsorption is an energy-efficient process |
|
Operational Cost |
High, due to specialized electrodes (e.g., BDD) and energy consumption |
Moderate, as it relies on consumable metal electrodes |
Low, as electrodes can be regenerated |
|
Scalability |
Challenging for large-scale applications due to energy demand |
Scalable for industrial and municipal treatment |
Scalable but requires optimization for long-term use |
|
Combination Potential |
Can be combined with filtration and adsorption for enhanced treatment |
Often used with filtration to remove coagulated PFAS |
Works well with EO for complete PFAS degradation |
|
Environmental Impact |
Minimal, as it fully degrades PFAS |
Can produce sludge requiring disposal |
Low, as it does not generate toxic byproducts |
PFAS’s electrostatic properties make this technique especially effective. High-surface-area electrodes—such as carbon aerogels, graphene-based materials, or modified activated carbon—trap PFAS through electrostatic attraction and hydrophobic interactions (Fig. 5) [10]. The process is reversible, enabling electrode regeneration and reducing waste and costs. A key advantage of electrosorption is its energy efficiency and ability to remove PFAS without additional chemical reagents. When combined with electrochemical oxidation (EO) or other treatments, it boosts overall PFAS degradation, making it a promising solution for sustainable water purification. Ongoing research aims to optimize electrode materials, voltage, and operating conditions for large-scale use. Table 1 shows a comparative analysis of three electrochemical treatment processes.
Conclusion and Future Prospects
Electrochemical methods show promise for PFAS remediation, each with trade-offs: EO offers effective degradation but is energy-intensive; EC efficiently removes PFAS but produces secondary waste; Electrosorption is energy-efficient but requires additional treatment for complete degradation.
Optimizing electrochemical PFAS treatment depends on specific goals, contaminant properties, and operational conditions. Hybrid approaches—like combining electrosorption with EO—can improve large-scale remediation. Advances in electrode materials, efficiency, and integration with adsorption or membrane systems may enhance cost-effectiveness and scalability. With increasing regulatory pressure and public concern, PFAS remediation remains a critical challenge. Ongoing research and innovation are key to securing cleaner water and a safer environment for future generations.
Super-Fast Charging Sodium-Ion Battery
Indian scientists at the Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR) in Bengaluru have developed a next-generation sodium-ion battery that charges to 80 % in just 6 minutes and lasts over 3,000 cycles. Developed by Prof. Premkumar Senguttuvan and Biplab Patra, the battery features a Nasicon-type structure (Nasicon: Sodium (Na) Super Ionic Conductor) and a specially engineered anode (Na1·0V0·25Al0·25Nb1·5(PO4)3). It incorporates nanoscale particle design, carbon coating, and aluminium doping to enhance ion transport, electrical conductivity, and structural stability, thereby ensuring long-term performance. With rapid charging, extended cycle life, and improved safety over lithium-ion systems, the battery relies on abundant, locally sourced sodium, offering an eco-friendly and scalable solution for EVs, solar grids, and rural electrification—supporting India’s push for technological self-reliance and potentially revolutionising its energy storage sector.
https://www.pib.gov.in/PressReleasePage.aspx?PRID=2129649
Zwitterionic Coatings for Blood-Contacting Medical Device
Blood-contacting devices like stents and heart valves face issues such as protein adhesion, which can cause thrombosis and device failure. Current heart valve implants often last less than a decade due to degradation and complications. Zwitterion-coated materials present a promising solution.
Zwitterions–molecules with both positive and negative charges but an overall neutral charge–form a strong hydration layer that repels proteins, reducing clot formation and extending implant life. Found naturally in cell membranes, they help maintain smooth blood flow. Compared to traditional hydrophilic coatings like polyethylene glycol (PEG), superhydrophilic zwitterionic polymers provide superior resistance to protein adsorption. A nanometre-thin coating can protect both coated and adjacent areas. However, optimizing polyzwitterions remains challenging due to interchain interactions. Research into hydrogen bonding and ionic interactions in zwitterionic brushes is advancing their antibiofouling performance, offering valuable potential for next-generation medical devices.
S. Talebian; M. Crago et al.: The interplay between grafting density and protein biofouling of polymer brushes: Curious case of polyzwitterions, Cell Biomaterials, 1, no. 1 (2025) 100005. doi: 10.1016/j.celbio.2024.100005
References:
[1] A. Bhattacharya; J. Fathima et al.: Advances in bioremediation strategies for PFAS-contaminated water and soil, Soil Environ. Health, 3, no. 1 (2025) 100126. doi; 10.1016/j.seh.2024.100126
[2] B.T.-W. Tan; N.H.H. Abu Bakar; H.L. Lee: Electrochemical methods for treatment of per- and polyfluoroalkyl substances (PFAS): A review, J. Environ. Chem. Eng., 13, no. 1 (2025) 114990. doi: 10.1016/j.jece.2024.114990
[3] F. Ajam; A. Khourshidi et al.: Per-and polyfluoroalkyl degradation in a hybrid dielectric barrier discharge plasma and electrooxidation system through involving more reactive species by air and water circulation, J. Hazard. Mater., 488 (2025) 137287. doi: 10.1016/j.jhazmat.2025.137287
[4] Z. Habib; M. Song et al.: Overview of per- and polyfluoroalkyl substances (PFAS), their applications, sources, and potential impacts on human health, Pollutants, 4, no. 1 (2024) 136-152. doi: 10.3390/pollutants4010009
[5] C. Gomri; E. Makhoul et al.: Electrochemical advanced oxidation combined to electro-Fenton for effective treatment of perfluoroalkyl substances “PFAS” in water using a Magnéli phase-based anode, Nanoscale Adv., 7, no. 1 (2024) 261-268. doi: 10.1039/d4na00626g
[6] N. Sharma; V. Kumar et al.: A comprehensive review on the need for integrated strategies and process modifications for per- and polyfluoroalkyl substances (PFAS) removal: Current insights and future prospects, Case Stud. Chem. Environ. Eng., 9 (2024) 100623. doi: 10.1016/j.cscee.2024.100623
[7] A. Kumar, A.K. Varma: Chapter 24 - Electrochemical Approaches with Novel Electrodes to Treat Emerging Contaminants in Water and Water Streams, In Emerging Trends and Advances in Microbial Electrochemical Technologies, A.K. Yadav, P. Srivastava, M.T. Noori, Y. Zhang (Editors), Elsevier, 2024, 681-708. doi: 10.1016/B978-0-443-15557-4.00016-2
[8] K. Sivagami; P. Sharma et al.: Electrochemical-based approaches for the treatment of forever chemicals: Removal of perfluoroalkyl and polyfluoroalkyl substances (PFAS) from wastewater, Sci. Total Environ., 861 (2023) 160440. doi: 10.1016/j.scitotenv.2022.160440
[9] H. Shi; S.-Y.D. Chiang et al.: An electrocoagulation and electrooxidation treatment train to remove and degrade per- and polyfluoroalkyl substances in aqueous solution, Sci. Total Environ., 788 (2021) 147723. doi: 10.1016/j.scitotenv.2021.147723
[10] H.N. Hussain; M.I. Jilani et al.: Advances in the removal of Polyfluoroalkyl Substances (PFAS) from water using destructive and non-destructive methods, Green Anal. Chem., 12 (2025) 100225. doi: 10.1016/j.greeac.2025.100225