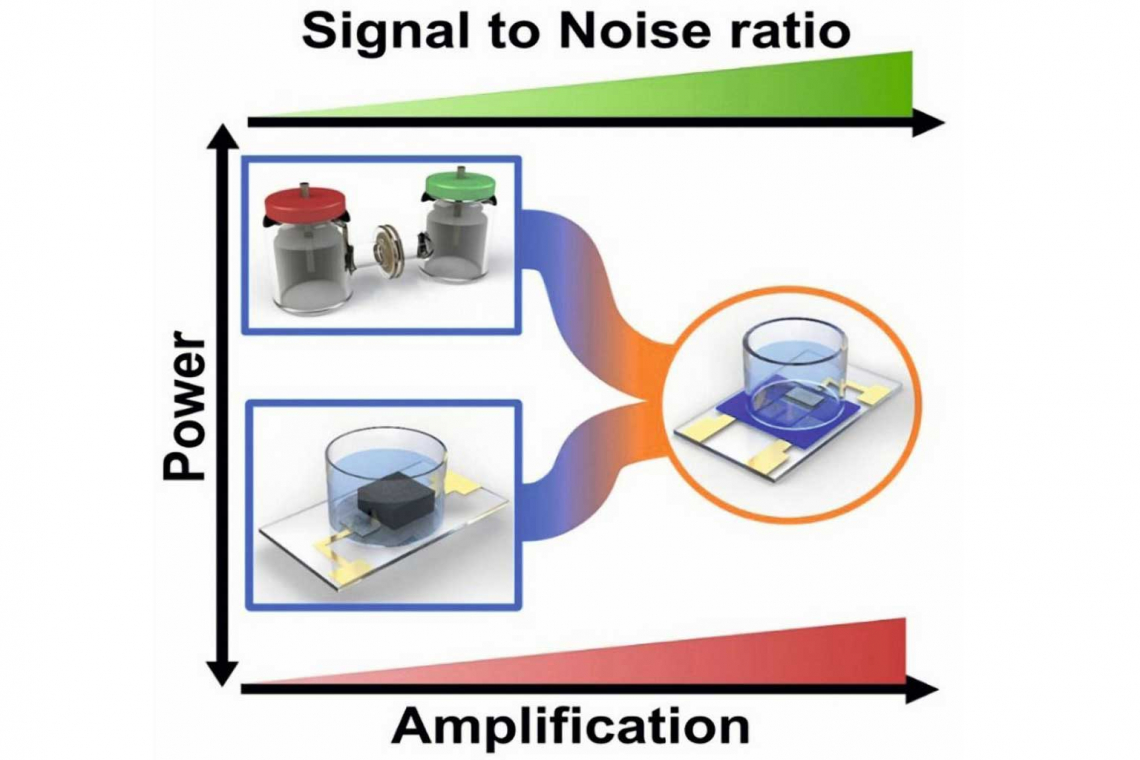
Organic Electrochemical Transistors Boost Bio-electronic Sensor Sensitivity
Biological and microelectronic systems both rely on electrons for information and energy transfer, but their integration faces challenges due to low microbial currents and environmental incompatibilities. This research presents a breakthrough in bioelectronic sensing, significantly enhancing the sensitivity of enzymatic and microbial fuel cells using organic electrochemical transistors (OECTs). By amplifying electrical signals by three orders of magnitude, this approach improves signal-to-noise ratios and enables highly sensitive, low-power biosensors for health and environmental monitoring. A graphical summary is shown in Figure 1.
Traditional biosensors, which depend on direct interactions between target biomolecules and the sensor device, often face limitations in incompatible electrolyte environments, making it a major challenge to design bioelectronic sensing systems that maintain performance across varying chemical conditions. This research circumvents that challenge by electronically coupling fuel cells with OECTs instead of directly introducing biomolecules into the sensor, enabling broader functionality. By keeping the OECT and fuel cell separate, optimal conditions are maintained for both components while still achieving powerful signal amplification. OECTs are thin-film transistors that operate in aqueous environments and have gained attention for their high sensitivity and low-voltage operation. In this research, OECTs were integrated with two types of biofuel cells – enzymatic and microbial – to enhance performance. Enzymatic fuel cells utilize glucose dehydrogenase to catalyse glucose oxidation, generating electricity, while microbial fuel cells rely on electroactive bacteria to metabolize organic substrates and produce current. The OECTs were then coupled with these fuel cells in two distinct configurations: a cathode-gate configuration and an anode-gate configuration.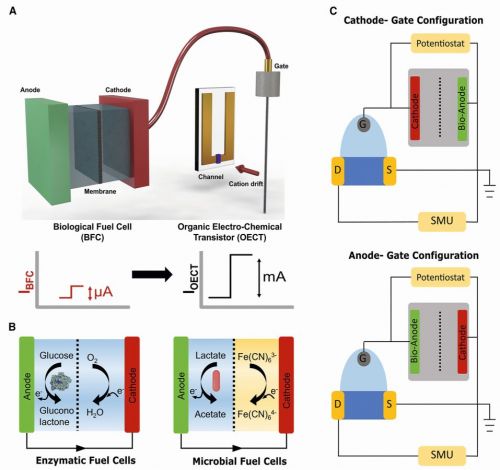 Fig. 2: Framework of OECTs coupled to biological fuel cells: A) Schematic illustrating OECT coupling with enzymatic fuel cells (EFCs) or microbial fuel cells (MFCs) for signal amplification, where small fuel cell currents translate into significantly larger OECT current changes. B) Schematics of EFC and MFC fuel cells. C) OECTs, biased via a source measure unit (SMU), can be configured in anode-gate or cathode-gate modes with EFCs or MFC
Fig. 2: Framework of OECTs coupled to biological fuel cells: A) Schematic illustrating OECT coupling with enzymatic fuel cells (EFCs) or microbial fuel cells (MFCs) for signal amplification, where small fuel cell currents translate into significantly larger OECT current changes. B) Schematics of EFC and MFC fuel cells. C) OECTs, biased via a source measure unit (SMU), can be configured in anode-gate or cathode-gate modes with EFCs or MFC
OECTs amplify signals from biofuel cells by factors ranging from 1,000 to 7,000, far exceeding traditional electrochemical techniques, which typically achieve only 10- to 100-fold enhancements. The cathode-gate con-figuration provided the highest amplification, especially with a specific polymer as the channel material, while the anode-gate configuration also showed strong amplification but faced potential degradation at higher fuel cell currents. Beyond boosting signal strength, OECTs reduced background noise, resulting in clearer, more reliable measurements. Even minor electrochemical changes were translated into large, detectable signals, enabling superior biomolecule and contaminant detection. Framework of OECTs coupled to biological fuel cells is shown in Figure 2.
This scalable technology has wide-ranging applications, demonstrated by a miniaturized system on a single glass slide, proving its feasibility for portable biosensors. One of its most promising uses is arsenite detection, a critical need for water safety. The research team engineered E. coli with an arsenite-responsive electron transfer pathway, enabling detection of concentrations as low as 0.1 micromoles per litre with a clear OECT-amplified response. Beyond water safety, this system holds potential for wearable health monitoring. A modular lactate sensor, incorporating microbial fuel cells and a cathode-gate configuration with Shewanella oneidensis MR-1, achieved over 1,000-fold signal amplification. It successfully converted microampere signals into milliampere-level outputs, offering a powerful and efficient tool for monitoring muscle fatigue in athletes, medical patients, and soldiers without the need for complex electronics.
Optimizing performance requires a clear understanding of power dynamics between OECTs and fuel cells. Researchers identified two key operational modes: power-mismatched, where the fuel cell generates less power than the OECT requires, resulting in higher sensitivity but operating near short-circuit conditions, and power-matched, where the fuel cell provides sufficient power for stable, accurate readings. Fine-tuning these interactions allows for sensor designs specifically tailored to applications in medical diagnostics and environmental monitoring.
R. Saxena; X. Zhang et al.: Amplification of enzymatic and microbial fuel cells using organic electrochemical transistors, Device, 3, no. 6, (2025) 100714. doi: 10.1016/j.device.2025.100714
https://www.cell.com/device/pdf/S2666-9986(25)00027-4.pdf
Green Hydrogen Peroxide Production from Air Using Metal-Air Batteries
Hydrogen peroxide (H2O2) is widely used as a bleach, disinfectant, and oxidizing agent. However, its industrial production is energy-intensive and relies on rare and precious metal catalysts, making it costly and environmentally unfriendly. Researchers at the Indian Institute of Science, Bengaluru (IISc-BLR), India have developed a sustainable, onsite production strategy using a Zn-air battery and a photoelectrochemical cell. Zinc is a widely available, historically used element known for its affordability and abundance. In a zinc-air battery, zinc serves as the anode (negative electrode) and ambient air as the cathode (positive electrode). During discharge, oxygen from the air is reduced at the cathode, generating H2O2. This approach not only generates H2O2 efficiently but also enables the simultaneous degradation of industrial pollutants like toxic dyes. The schematic illustrating the operation of the Zn-Air battery and the dye degradation process is presented in Figure 3.
The electrochemical reduction of oxygen at the cathode can proceed via two pathways—one leading to H₂O₂ formation and the other to water. Controlling this reaction is key to maximizing H2O2 selectivity, achieved through chemical modifications in metal-free carbon-based catalysts. By fine-tuning the voltage, the system prioritizes H2O2 production while storing electrical energy within the battery.
![Fig. 3: Schematic of operation of Zn-Air battery and dye degradation [D.S. Jhawar] Fig. 3: Schematic of operation of Zn-Air battery and dye degradation [D.S. Jhawar]](/images/stories/Abo-2025-08/gt-2025-08-57.jpg) Fig. 3: Schematic of operation of Zn-Air battery and dye degradation [D.S. Jhawar]
Fig. 3: Schematic of operation of Zn-Air battery and dye degradation [D.S. Jhawar]
Using an optimally produced reduced graphene oxide (rGO) electrocatalyst@air-cathode, the Zn-air system achieves an impressive power density of 320 W/mgeo2 (geo = geometric area) and a high H2O2 production rate of 3.17 mol/mgeo2/h at 0.8 V. Systematic investigations reveal that specific functional groups (C–O–C, chemisorbed O2, C≐C) enhance H2O2 yield. The in situ generated superoxide (O2˙) and hydroxyl radicals (˙OH) efficiently degrade rhodamine B, a model textile dye pollutant, inside the Zn-air cell.
In a parallel approach, the same rGO catalyst functions as a photoelectrode in an H-type cell, significantly enhancing H2O2 production under visible light. This setup enables pollutant degradation five times faster than in the Zn-air cell at the same operating potential (0.8 V). Since H2O2 is colourless, its presence is confirmed by introducing a textile dye pollutant that degrades upon reaction, visually indicating successful H2O2 generation.
Despite the complexity of metal-air batteries, which involve solid, liquid, and gas phases, researchers believe this strategy is scalable and holds promise for broader applications, including electricity generation in remote locations. By integrating electrosynthesis with environmental remediation, this method offers a low-cost, energy-efficient, and sustainable alternative for H2O2 production.
A. Behera; A.J. Bhattacharyya: Employing a Zn‐air/photo‐electrochemical cell for in situ generation of H2O2 for onsite control of pollutants, Small Methods, 9, no. 4 (2025) 2401539. doi: 10.1002/smtd.202401539
Pyrene tetraone derivatives for long-life aqueous organic flow batteries
Multi-electron transfer molecules offer great potential to enhance energy density and reduce costs for aqueous organic flow batteries (AOFBs). However, increasing redox-active sites through extended conjugation often decreases molecular polarity, limiting solubility in electrolytes. To address this, researchers designed an asymmetrical pyrene-4,5,9,10-tetraone-1-sulfonate (PTO-PTS) monomer via a coupling oxidation-sulfonation reaction. This high-water-soluble derivative reversibly stores four electrons, achieving a theoretical electron concentration of 4M and stabilizing an intermediate semiquinone free radical. The extended conjugated structure enables reversible four-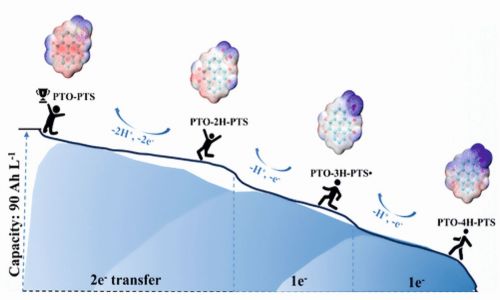 Fig. 4: Graphical illustration of AOFBs, PTO–PTSelectron transfer through enolization tautomerism, while the sulfonic acid group enhances charge density and hydrogen bonding, improving solubility. A graphical illustration is presented in Figure 4.
Fig. 4: Graphical illustration of AOFBs, PTO–PTSelectron transfer through enolization tautomerism, while the sulfonic acid group enhances charge density and hydrogen bonding, improving solubility. A graphical illustration is presented in Figure 4.
Applied to AOFBs, PTO–PTS achieved an ultra-high volumetric capacity of ~90 Ah/L and an energy density of 60 Wh/L. These batteries demonstrated nearly 100% capacity retention over 5,200 cycles in air, indicating excellent long-term stability. Additionally, the structure's stability at high temperatures allowed both symmetric and full cells to maintain performance over thousands of cycles at 60 °C, with durability spanning ~1,500 hours across a broad temperature range (10-60 °C). The outstanding cell performance and thermal stability of PTO–PTS highlight its promise for large-scale energy storage applications.
The exceptional cell performance and thermal stability of PTO–PTS underscore its potential for large-scale energy storage. AOFBs are promising for renewable energy integration and grid storage due to their inherent safety and the availability of abundant, tuneable organic redox-active molecules (ORAMs). However, commercialization is challenged by low energy density, poor stability at high concentrations, and high synthesis costs. Enhancing ORAMs to achieve both high energy density and ultra-stable cycling is crucial for advancing stationary energy storage. Increasing electron transfer can improve energy density and lower electrolyte costs, but multi-electron transfer ORAMs often struggle with a trade-off between stability and solubility.
G. Ge; C. Mu et al: Four-elec-tron-transferred pyrene-4,5,9,10-tetraone derivatives enabled high-energy-density aqueous organic flow batteries, J. Amer. Chem. Soc., 147, no. 6 (2025) 4790-4799. doi: 10.1021/jacs.4c12506
Sustainable Green Hydrogen Production from Sunlight and Seawater
Green hydrogen, produced by water electrolysis powered by renewables, is vital for decarbonizing hard-to-abate sectors. However, its scalability is limited by the need for large volumes of clean water – around 9 kg per kg of hydrogen – worsening global water scarcity. A hybrid solar distillation–water electrolysis (HSD-WE) system addresses this challenge by using seawater and sunlight. It combines photovoltaic (PV) and photothermal (PT) effects: high-energy photons generate electricity for electrolysis, while the remaining solar energy drives interfacial thermal distillation (Fig. 5). Waste heat from the PV panel purifies water in situ, reducing electrode fouling and corrosion without extra power input.
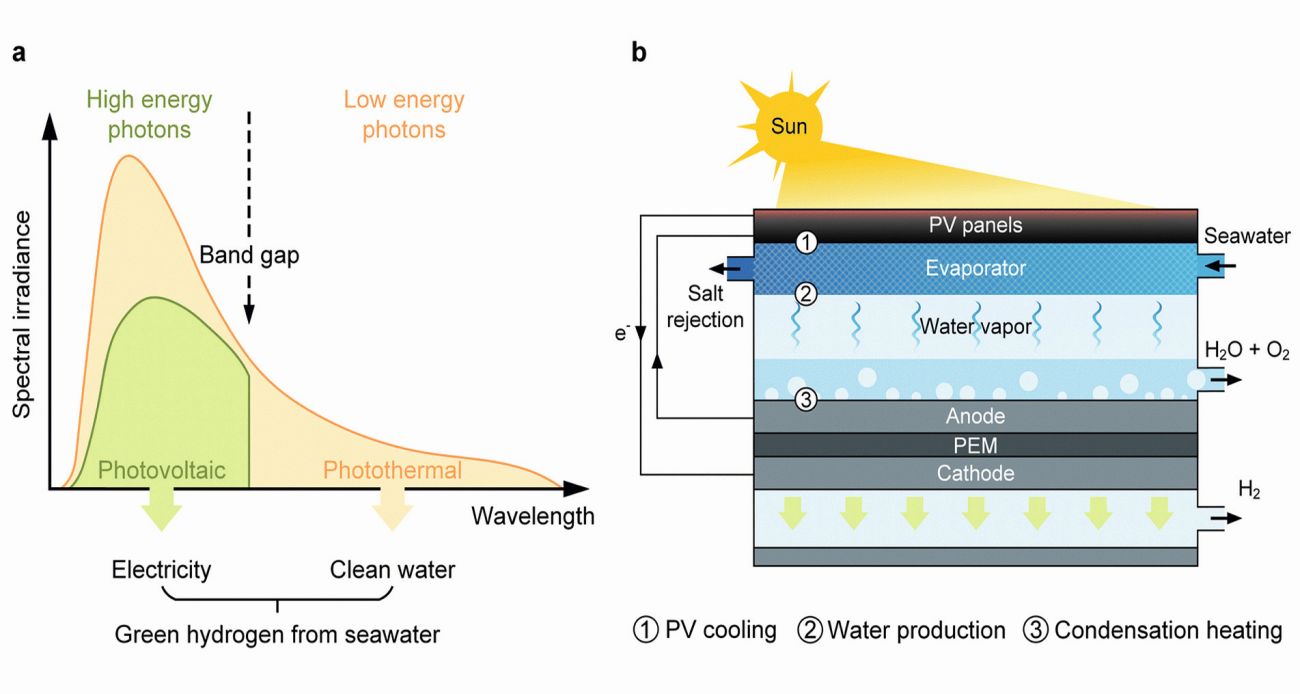 Fig. 5: Solar-powered green hydrogen production from seawater. a) Full-spectrum utilization, b) Schematic of the hybrid solar distillation-water electrolysis (HSD-WE) device
Fig. 5: Solar-powered green hydrogen production from seawater. a) Full-spectrum utilization, b) Schematic of the hybrid solar distillation-water electrolysis (HSD-WE) device
Tested under real seawater and sunlight conditions, the system achieves over 12 % solar-to-hydrogen efficiency, producing 35.9 L m–2 h–1 of hydrogen and 1.2 L m–2 h–1 of clean water. Its passive operation, low-cost materials, integrated PV cooling, and salt rejection enhance both performance and economic viability. This method offers a scalable, sustainable solution for hydrogen production in water-scarce, off-grid areas.
Wang, X; Gao, J. et al.: Over 12% efficiency solar-powered green hydrogen production from seawater, Energy Environ. Sci.,18, no.11 (2025) 5264-5276. doi: 10.1039/D4EE06203E