Photoelectrochemical (PEC) water splitting presents a sustainable and environmentally friendly means of generating hydrogen and potentially reducing dependence on fossil fuels. The p-type Cu2O semiconductor is a favorable option for a photocathode material due to its cost-effectiveness, widespread availability of the base materials, light-absorbing properties, and suitable energy band positions. This shown method is employed to create Cu2O photocathodes with enhanced PEC performance, featuring a significantly porous structure with a density ranging from 55 to 80 pores per square millimeter.
Optimizing angles, increasing surface
The surface of thin Cu2O films can be intentionally structured to optimize the angle for incoming light or the photon pathway. This strategic adjustment results in a substantial enhancement of light absorption while preserving a short electron diffusion length to the electrolyte. The practical method used in this work involves coating the walls of the pores with Cu2O as depicted in Figure 1. Furthermore, the modified structure increases the surface area tremendously, leading to a higher exposure of the photoactive sites, and accelerating the electrochemical reactions.Utilizing the electrodeposition method, which is both cost-effective and readily scalable, enables the production of a substantially uniform Cu2O film which thus can generate consistently high PEC performance. Moreover, this technique is adaptable for application on a three-dimensional porous substrate, offering the flexibility to finely control film thickness through adjustment of deposition parameters.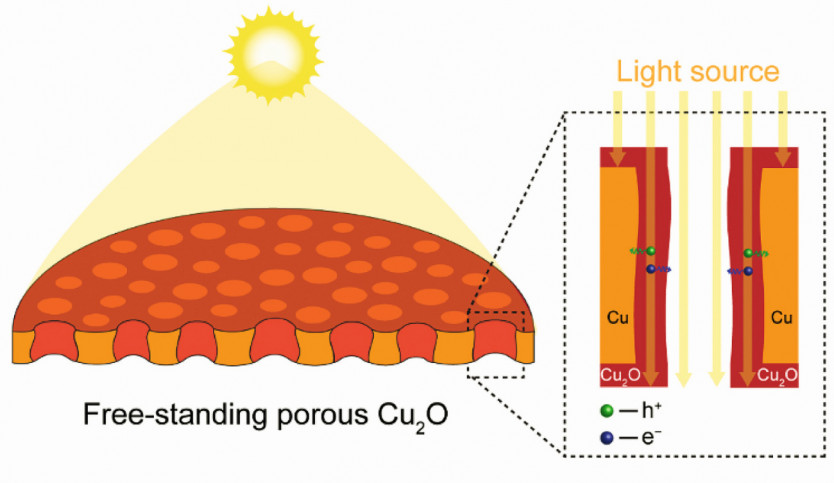 Fig. 1: Schematic representation of the Cu2O film covering a vertically oriented porous structure, aimed at improving light absorption while maintaining a short electron diffusion length
Fig. 1: Schematic representation of the Cu2O film covering a vertically oriented porous structure, aimed at improving light absorption while maintaining a short electron diffusion length
H2-bubbles in an acidic copper bath
A three-stage electrochemical procedure was implemented to produce a stable and highly porous copper metal framework as a substrate, which was subsequently coated with Cu2O film as depicted in Figure 2. In the initial stage, a dendritic porous copper structure with a large surface area was electrodeposited onto a flat copper substrate via a galvanostatic route. This process utilized dynamic hydrogen bubbles as a soft template in an acidic copper bath to create porous structures. Employing higher current densities (>1.5 A cm–2) for a short duration resulted in the production of more hydrogen bubbles, which had an elevated detachment rate. This led to improvements in pore distribution and a strong reduction of the pore sizes. However, at this stage due to the high current density, the pore walls predominantly consisted of dendritic copper branches which offer a large surface area but were mechanically too fragile for practical applications.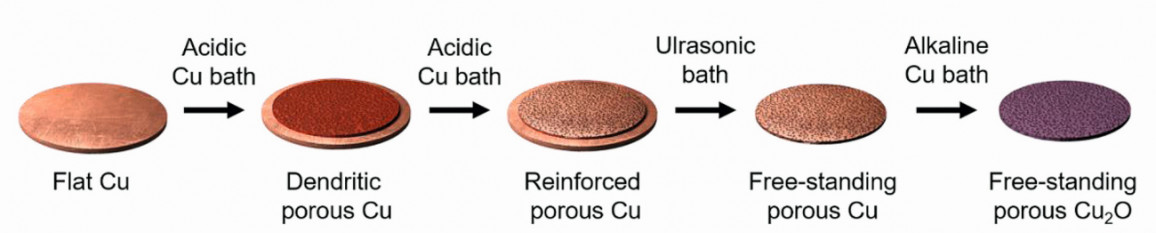 Fig. 2: Illustration depicting the synthesis process for creating a porous Cu2O photocathode
Fig. 2: Illustration depicting the synthesis process for creating a porous Cu2O photocathode
Reinforcing the porous structure
To address this issue, a secondary electrodeposition step, utilizing the same acidic bath but with a significantly lower current density (20 mA cm–2), was employed for at least 60–120 min to reinforce the porous structure and improve its mechanical stability. Interestingly the dendritic reinforcement at the boundary between the copper substrate and the porous copper layer was less efficient. As a result, the porous copper layer could be effortlessly detached from the substrate through a brief ultrasonication process, ultimately yielding a stable, free-standing porous copper framework. The pore size of the porous copper frameworks can be conveniently adjusted, with an average pore diameter ranging from 50 to 100 μm, simply by altering the electrodeposition duration (30–60 s) during the fabrication of the dendritic porous copper. A shorter duration results in a porous sample with smaller pores. Finally, to complete the whole synthesis process, a Cu2O film was electrodeposited at a fixed potential of -0.4 V vs. Ag/AgCl (sat. KCl) onto the porous copper framework in an alkaline copper bath containing 0.2 M CuSO4 (> 99.8 % copper sulfate, Honeywell Fluka) and 1 M Na3C6H5O7 2H2O (> 99 % trisodium citrate dihydrate, Sigma-Aldrich) as a complexing agent and saturated NaOH solution to increase the pH of the bath to 12. The alkaline bath was maintained at a temperature of 60 °C to ensure good crystallinity of the deposited Cu2O layer.
The thicker the copper oxide film, the weaker the photoactivity
Linear sweep voltammetry (LSV) was conducted to assess the photoactivity of the Cu2O photocathode in relation to its performance in PEC water splitting. The voltage sweep ranged from 0.45 to -0.15 V vs. RHE, and a Biologic potentiostat SP-240 was utilized to record the current at a scan rate of 5 mV s–1 in an 0.5 M Na2SO4 electrolyte (pH 6). These measurements were conducted under chopped light illumination, with alternating 5 s periods of light and darkness. The photoactivity of the Cu2O photocathodes exhibits a weakening trend as the thickness of the Cu2O film increases. Remarkably, the sample deposited for 5 minutes on a flat Cu substrate which has a thickness of ~500 nm displays the highest photocurrent density, approximately 1.25 mA cm–2 at 0 V vs. RHE, when subjected to 100 mW cm–2 with 1.5 AM filter illumination, alongside a low dark current of around -35 μA cm–2. The most favorable overall performance regarding PEC performance, however, was observed in the case of the free-standing porous Cu2O samples as shown in Figure 3. These samples yielded significant photocurrent densities, reaching -2.25 mA cm-2 (porous Cu 60 s) and -2.75 mA cm–2 (porous Cu 30 s) while still maintaining a low dark current of approximately -35 μA cm–2 at 0 V vs. RHE, representing an increase of 80 % and 120 % compared to the performance of the Cu2O film on a flat Cu substrate.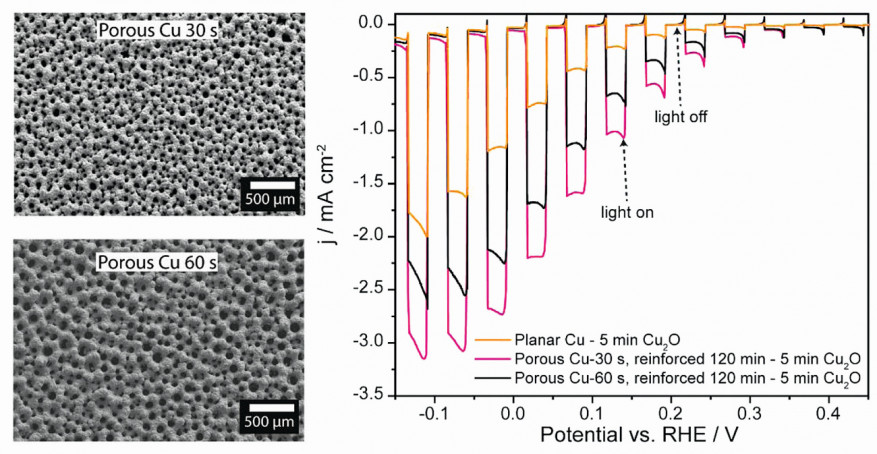 Fig. 3: Linear sweep voltammograms depict the photoelectrochemical performance of the Cu2O film on a flat copper surface as well as on two distinct varieties of free-standing porous copper substrates. These free-standing porous Cu samples were electrodeposited at -1.5 A cm–2 for 30 s (small pores) and 60 s (large pores) Graphics: TU Ilmenau
Fig. 3: Linear sweep voltammograms depict the photoelectrochemical performance of the Cu2O film on a flat copper surface as well as on two distinct varieties of free-standing porous copper substrates. These free-standing porous Cu samples were electrodeposited at -1.5 A cm–2 for 30 s (small pores) and 60 s (large pores) Graphics: TU Ilmenau
Summary
In conclusion, a preparation route was established to produce high-performance Cu2O photocathodes through electrochemical deposition processes. Establishing a homogenous Cu2O film layer is crucial for reducing the dark current and enhancing photoelectrochemical performance. This objective is achievable through the utilization of the suggested electrodeposition methods. When combined with a thin layer of Cu2O, the free-standing porous structure demonstrated a remarkable influence on the photoelectrochemical performance, primarily attributable to its enlarged active surface area, improved light absorption, and reduced electron diffusion length to the surface.
Dr. Mario Kurniawan hat mit diesem Forschungsthema den Nasser-Kanani-Preis gewonnen. Die Auszeichnung wurde beim Ulmer Gespräch im Mai 2023 verliehen.