Plasma electrolytic oxidation imparts improved microhardness, corrosion and wear resistance; tribological and heat radiation characteristics in many aggressive environments, and favourable topography for cellular proliferation [1–8]. The process is carried out in mild aqueous alkaline electrolytes [9–18] and as the coating also grows inwards, an extremely adhesive oxide coating on the substrate is formed. The process provides uniform coverage on complex shapes with well-controlled, predictable growth and superior edge protection. The non-columnar structure of PEO coatings is less susceptible to wear with lower fatigue debit. The process is widely used for growing thick, hard, largely crystalline oxide coating on Al, Mg and Ti alloys [2, 11, 19–27]. The technological development, microstructural characteristics, biocompatibility and applications of PEO for general as well as specific objectives are reviewed elsewhere [1, 5, 12, 19, 28–43]. PEO coatings are used for diverse applications:
- Decorative applications: Depending on substrate composition, electrolyte formulation and processing parameters, the colour of the coatings can be whitish, gray or black. Further by designing the parameters for the porosity of PEO coating, the absorption of colouring agents, dyes, paints, etc. can be enhanced.
- Superior corrosion resistance: PEO coating provides far superior corrosion resistance than the conventional anodizing.
- Superior mechanical properties: Owing to the possession of very high hardness and superior tribological performance, the PEO coatings are used to improve the wear and friction resistance.
- Thermal control applications: As the thermal conductivity of the PEO oxide layer is very low, it can be used as a thermal barrier / thermal shock resistance layer [44, 45]. In addition, the PEO coatings can also be blackened to improve their heat absorptance characteristics for thermal control applications.
- Electrical appliances: As the PEO layer has very high dielectric strength, it is very useful for electrical insulation. The dielectric breakdown occurs at a field of ~10 V per micron. In addition, the PEO coating is very hard, it is suitable for implantation on the inner surfaces of conic, cavital or cylindrical areas.
- Chemical industries: PEO coatings on titanium can be used in chemical industries due to their resistance to acidic and alkaline solutions, as well as for the photo-activity of titania (TiO2) [46].
- Biomedical applications: PEO coated titanium components are used in biomedical devices, biodegradable materials and dental implants [47–51].
- Super hydrophobic characteristics: The micro- and nanosized TiO2 particles have been utilized to improve the surface roughness (water contact angle, WCA below 10°) for self-cleaning, antifogging and related applications [52].
- The PEO coatings can be impregnated with polymers such as PTFE to modify their properties for specific applications.
Process Set-Up
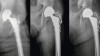
An image of a typical plasma electrolytic oxidation plant is shown in Figure 1. A schematic set-up of PEO process is represented in Figure 2. It consists of a stainless-steel tank which contains the electrolyte and a suitable high power supply source. The job is immersed in the aqueous electrolyte and is electrically connected in the electrochemical cell, so as to become an anode. The other counter-electrode (cathode) is typically made from an inert material such as stainless steel, and often consists of the wall of the bath itself. The electrolyte is stirred well either mechanically or by air agitation and cooled through a cold-water circulation unit. The PEO coating is formed by polarizing the titanium part to the dielectric breakdown voltage in a suitable electrolyte. The process is similar to anodizing, but it employs higher potentials, so that discharges occur which leads to partial fusion of an oxide film and the resulting plasma modifies the structure of the layer [44, 45, 54–60]. The mechanism of the PEO process consists of complex physical, chemical, electrochemical and plasma thermo-chemical reactions. Oxide film formation, dissolution of primary film and gas evolution on anode surface constitute the major phenomena.
The process can employ the continuous direct current or pulsed direct current or alternating pulses (pulsed unipolar and pulsed bi-polar). Since the number of electrical parameters that can be regulated in a direct current mode is limited, it is not commonly adopted. Further, the use of AC sources is typically limited by the possible output power (≤ 10 kW) and frequency. The application of pulsed DC current mode has greatly enabled tailoring of PEO coating microstructure and characteristics by efficiently modifying the surface discharge characteristics by simple adjustments of electrical parameters like wave form, pulse width, duty cycle, frequency, etc. This has led to the rapid development of PEO technique.
A unipolar current mode has only the positive component whereas a bipolar current mode consists of both a positive component and a negative component. The use of a hybrid mode of current i.e., a combination of pulsed unipolar and bipolar current modes has also been reported.
Process Mechanism
The PEO coatings on Ti can be obtained with silicate, phosphate, aluminate, silicate-phosphate, phosphate-aluminate based electrolytes. The PEO coating of thicknesses of ~200 μm can be generally achieved on Ti alloy with the corresponding micro hardness values in the range of 300–1100 HV [61]. Table 1 shows details of typical baths:
The chemical reactions for PEO coating of titanium include; anodic dissolution of titanium, formation of titania, and oxygen evolution. The anions (OH– and AlO2–, Al(OH)4–, PO43–, SiO32–) depending on the electrolyte composition participate in the reaction at the oxide/electrolyte interface. The chemistry of the whole process can be represented with the following general equations [46].
Metal-oxide interface: anodic process
Ti → Ti4+ + 4e–
4OH– → O2↑ + 2 H2O+ 4e–
Oxide-electrolyte interface
Ti4+ + 4OH– → TiO2 + 2H2O
3Ti4+ + 10OH– → Ti3O5 + 5H2O
Ti4+ + 4AlO2– → TiO2 + 2Al2O3
Ti4+ + Al(OH)4– → TiO2 + Al2O3 + Al(OH)3 + 5H2O
TiO2 + Al2O3 → Al2TiO5
3Ti4+ + 4PO43−→ Ti3(PO4)4
Ti4+ + SiO32− + 2OH– → TiSiO4 + H2O
Ti4+ + 2SiO32− → Ti(SiO3)2
|
Electrolytes |
Composition (mol/L) |
pH |
Conductivity (mS/cm) |
Coating Thickness (μm) |
|
|
1. Silicate |
Na2SiO3·9H2O |
0.05 |
12.69 |
16.86 |
83.2 |
|
2. Phosphate |
Na3PO4·12H2O |
0.05 |
12.23 |
12.69 |
20.7 |
|
3. Mixed Electrolyte |
Na2SiO3·9H2O |
0.025 |
12.53 |
14.60 |
25.2 |
|
Na3PO4·12H2O |
0.025 |
||||
|
Operating conditions: Anodic current density: 8 A/dm2, Temperature: 15–25 °C, Time: 60 minutes |
|||||
Tab. 1: Details of typical PEO process on Ti alloys [62]
Aliasghari et al. [63] used the phosphate/silicate electrolyte: sodium silicate (SG,1.5): 10.5 g/L, phosphoric acid: 2 ml/L, KOH: 2.8 g/L; conductivity: 10.2 mS/cm; pH: 12.2; 25 °C, 50 A/dm2; 50 Hz; duty cycle: 50 %; 0 to 310 V for PEO coatings on Ti6Al4V alloy. A coating thickness of 40–50 µm was produced in 60 minutes. Li et al. [64] fabricated 110 µm thick PEO coating on TiAl alloy by the alternating-current in silicate electrolyte. Microhardness of the coating was about three times higher than that of TiAl substrate. The wear rate of coating was only 1/10 of TiAl substrate. Corrosion current density of the coated TiAl alloy was greatly reduced.
Microstructure
The microstructure and phase content of the PEO coatings are related to the process parameters which ultimately influence the wear and corrosion resistance [65, 66]. The PEO coating consists of a porous outer layer with pore diameter ranging from 3 to 8 µm (Fig. 3) [67], dense intermediate layer and a thin inner layer [61]. The surface morphologies are characterized by many micropores, microcracks and dimples [68]. The coatings have non-columnar structure, and are dense near the substrate interface and intermediate layer. The porosity is more near the surface around discharge channels. The micropores are formed by molten oxide and gas bubbles thrown out of micro-arc discharge channels and the cracks are resulted from the thermal stress due to the rapid solidification of the molten oxide in the relatively cold electrolyte. Porous structures in anodic coatings are potentially favourable to some applications where the addition of other material is required to improve the surface properties, for example a solid lubricant material, such as polytetrafluoroethylene (PTFE), or a secondary coating.
![Fig. 3: SEM micrographs of PEO coating on Ti (a) overview and (b) detail [67]](/images/stories/Abo-2023-09/gt-2023-09-075.jpg) Fig. 3: SEM micrographs of PEO coating on Ti (a) overview and (b) detail [67]
Fig. 3: SEM micrographs of PEO coating on Ti (a) overview and (b) detail [67]
PEO technique provides a possibility for the wide variation of composition and structure of the surface oxide film and thus attracts special interest for the corrosion protection and the optimization of friction and wear of titanium alloys. The process also allows the fabrication of relatively rough, micro-porous, thick, titanium oxide coatings containing bioactive compounds (such as hydroxyapatite, calcium phosphate or calcium titanate) to enhance the osseointegration for biomedical applications [69–73].
Solar Selective / High Emittance PEO Coatings
Studies on some solar selective / high emittance PEO coatings on titanium materials for thermal control applications in space environments are reported. Yao et al. [74] fabricated solar reflector, low absorbance and high emissivity, PEO coatings on Ti6Al4V alloy with silicate electrolytes. The concentration of silicate and the applied current density influence the thickness and the roughness of the coatings, and consequently the thermal control properties. The optimum results were exhibited with 10 g/L Na2SiO3 and 1 g/L NaH2PO2·H2O at a current density of 10 A/dm2, frequency: 500 Hz; time: 30 minutes; where a coating thickness of 80–100 µm resulted in a hemispherical emittance of 0.92 and a solar absorptance of 0.39 at 318 K. The coating is mainly composed of O, Si, Ti, P and Na. It was not well crystallized and consisted of a large number of amorphous silicates.
A high-emittance PEO process on Ti6Al4V for spacecraft application was obtained with electrolytes containing sodium silicate and sodium hypophosphate [75]. Average εir and αs of 5–9 μm thick coating were recorded as 0.85, and 0.76, respectively. The PEO coating was found to be hydrophobic (contact angle ~108°). The characteristic nanohardness (H) and Young’s modulus (E) values were 2.59 GPa and 81.45 GPa, respectively. The corrosion resistance of the PEO coating was evaluated by potentiodynamic polarization and Electrochemical impedance spectroscopy (EIS). The total impedance and corrosion current density (icorr) were in the order of 1 MΩ and 10-8 µA.cm–2, respectively. The results indicate superior corrosion resistance of the present PEO coating compared to similar coatings reported earlier in the literature.
REFERENCES
[1] B.L. Jiang; Y.M. Wang: Chapter 5, Plasma Electrolytic Oxidation Treatment of Aluminium and Titanium Alloys in Surface Engineering of Light Alloys. Aluminium, Magnesium and Titanium Alloys, H. Dong (Editor), Woodhead Publishing, Elsevier, (2010), 110–154, doi: 10.1533/9781845699451.2.110
[2] A. Lugovskoy; M. Zinigrad: Chapter 4, Plasma Electrolytic Oxidation of Valve Metals, in Materials Science-Advanced Topic, Y. Mastai (Editor), IntechOpen, (2013), 85–102, doi: 10.5772/54827
[3] M. Echeverry-Rendon; O. Galvis; D. Quintero Giraldo; J. Pavon; J.L. Lopez-Lacomba; E. Jimenez-Pique; M. Anglada; S.M. Robledo; J.G. Castano; F. Echeverria: Osseointegration improvement by plasma electrolytic oxidation of modified titanium alloys surfaces, J. Mater. Sci. Mater. Med., 26(2015)2, 72, doi: 10.1007/s10856-015-5408-4
[4] A.R. Rafieerad; M.R. Ashra; R. Mahmoodian; A.R. Bushroa: Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: A review paper, Mater. Sci. Eng. C Mater. Biol. Appl., 57(2015), 397–413, doi: 10.1016/j.msec.2015.07.058
[5] S. Stojadinovic; N. Radic; B. Grbic; S. Maletic; P. Stefanov; A. Pacevski; R. Vasilic: Structural, photoluminescent and photocatalytic properties of TiO2:Eu3+ coatings formed by plasma electrolytic oxidation, Appl. Surf. Sci., 370(2016), 218–228, doi: 10.1016/j.apsusc.2016.02.131
[6] M. Echeverry-Rendon; O. Galvis; R. Aguirre; S. Robledo; J.G. Castano; F. Echeverria: Modification of titanium alloys surface properties by plasma electrolytic oxidation (PEO) and influence on biological response, J Mater Sci Mater Med., 28(2017)11, 169, doi: 10.1007/s10856-017-5972-x
[7] M.L. Grilli; D. Valerini; A.E. Slobozeanu; B.O. Postolnyi; S. Balos; A. Rizzo; R.R. Piticescu: Critical raw materials saving by protective coatings under extreme conditions: A review of last trends in alloys and coatings for aerospace engine applications, Materials, 14(2021)7, 1656, doi: 10.3390/ma14071656
[8] X. Lu; T. Zhang; Y. Lv; X. Zhang; Z. Dong: Corrosion behviour of micro-arc oxidized titanium in NaCl solution with H2O2 and albumin, Mater. Chem. Phys., 276(2022), 125376, doi: 10.1016/j.matchemphys.2021.125376
[9] S. Shrestha; B.D. Dunn: Advanced plasma electrolytic oxidation treatment for protection of lightweight materials and structures in a space environment, Surface World, (2007), 40–44
[10] C. Mısırlı; M. Sahin; U. Sozer: Chapter 4, Effect of Micro Arc Oxidation Coatings on the Properties of Aluminium Alloys in Aluminium Alloys- New Trends in Fabrication and Applications, Zaki Ahmad (Editor), IntechOpen, (2013), 107–120, doi: 10.5772/53135
[11] V. Dehnavi; B.L. Luan; X.Y. Liu; D.W. Shoesmith; S. Rohani: Correlation between plasma electrolytic oxidation treatment stages and coating microstructure on aluminum under unipolar pulsed DC mode, Surf. Coat. Technol., 269(2015), 91–99, doi: 10.1016/j.surfcoat.2014.11.007
[12] R.O. Hussein; X. Nie; D.O. Northwood: An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing, Electrochim. Acta, 112(2013), 111–119, doi: 10.1016/j.electacta.2013.08.137
[13] Y. Pan; C. Chen; D. Wang; X. Yu: Microstructure and biological properties of micro‐arc oxidation coatings on ZK60 magnesium alloy, J. Biomed. Mater. Res. B, 100(2012), 1574–1586, doi: 10.1002/jbm.b.32726
[14] H. Guo; M. An: Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate-fluoride solutions and evaluation of corrosion resistance, Appl. Surf. Sci., 246(2005)1-3, 229–238, doi: 10.1016/j.apsusc.2004.11.031
[15] A. Ghasemi; V.S. Raja; C. Blawert; W. Dietze; K.U. Kainer: Study of the structure and corrosion behavior of PEO coatings on AM50 magnesium alloy by electrochemical impedance spectroscopy, Surf. Coat. Technol., 202(2008), 3513–3518, doi: 10.1016/j.surfcoat.2007.12.033
[16] J. Liu; Y. Lu; X. Jing; Y. Yuan; M. Zhang: Characterization of plasma electrolytic oxidation coatings formed on Mg-Li alloy in an alkaline silicate electrolyte containing silica sol, Mater. Corros., 60(2009), 865–870, doi: 10.1002/maco.200805204
[17] X. Wu; P. Su; Z. Jiang; S. Meng: Influences of current density on tribological characteristics of ceramic coatings on ZK60 Mg alloy by plasma electrolytic oxidation, ACS Appl. Mater. Interfaces, 2(2010)3, 808–812, doi: 10.1021/am900802x
[18] J. Cai; F. Cao; L. Chang; J. Zheng; J. Zhang; C. Cao: The preparation and corrosion behaviors of MAO coating on AZ91D with rare earth conversion precursor film, Appl. Surf. Sci., 257(2011)8, 3804–3811, doi: 10.1016/j.apsusc.2010.11.153
[19] A.L. Yerokhin; X. Nie; A. Leyland; A. Matthews; S.J. Dowey: Plasma electrolysis for surface engineering, Surf. Coat. Technol., 122(1999)2–3, 73–93, doi: 10.1016/S0257-8972(99)00441-7
[20] Y. Zhang; W. Fan; H.Q. Du; Y.W. Zhao: Plasma electrolytic oxidation coatings for aluminum alloys, Mater. Performance, 56(2017)9, 38–41, www.researchgate.net/publication/320694309[21] E. Matykina; R. Arrabal; M. Mohedano; B. Mingo; J. Gonzalez; A. Pardo; M.C. Merino: Recent advances in energy efficient PEO processing of aluminium alloys, Trans. Nonferrous Met. Soc. China, 27(2017)7, 1439–1454, doi: 10.1016/S1003-6326(17)60166-3
[22] J. Martin; A. Nomine; V. Ntomprougkidis; S. Migot; S. Bruyere; F. Soldera; T. Belmonte; G. Henrion: Formation of a metastable nanostructured mullite during plasma electrolytic oxidation of aluminium in soft regime condition, Mater. Des., 180(2019), 10797, doi: 10.1016/j.matdes.2019.107977
[23] F.C. Walsh; C.T.J. Low; R.J.K. Wood; K.T. Stevens; J. Archer; A.R. Poeton; A. Ryder: Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys, Trans. Inst. Met. Finish., 87(2009)3, 122–135, doi: 10.1179/174591908X372482
[24] E.A. Akbar; M.A. Qaiser; A. Hussain; R.A. Mustafa; D. Xiong: Surface modification of aluminum alloy 6060 through plasma electrolytic oxidation, Int. J. Eng. Works, 4(2017)6, 114–123, hal.archives-ouvertes.fr/hal-01533006/document
[25] L.O. Snizhko; A.L. Yerokhin; A. Pilkington; N.L. Gurevina; D.O. Misnyankin; A. Leyland; A. Metthews: Anodic processes in plasma electrolytic oxidation of aluminum in alkaline solutions, Electrochim. Acta, 49(2014)13, 2085–2095, doi: 10.1016/j.electacta.2003.11.027
[26] X. Liu; S. Wang; N. Du; Q. Zhao: Evolution of the three-dimensional structure and growth model of plasma electrolytic oxidation coatings on 1060 aluminum alloy, Coatings, 8(2018)3, 105, doi: 10.3390/coatings8030105
[27] G.P. Wirtz; S.D. Brown; W.M. Kriven: Ceramic coatings by anodic spark deposition, Mater. Manuf. processes, 6(1991)1, 87–115, doi: 10.1080/10426919108934737
[28] X. Lu; M. Mohedano; C. Blawert; E. Matykina; R. Arrabal; K.U. Kainer; M.L. Zheludkevich: Plasma electrolytic oxidation coatings with particle additions - A review, Surf. Coat. Technol., 307(2016), 1165–1182, doi: 10.1016/j.surfcoat.2016.08.055
[29] T.W. Clyne; S.C. Troughton: A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals, Int. Mater. Rev., 64(2019)3, 127–162, doi: 10.1080/09506608.2018.1466492
[30] M. Toorani; M. Aliofkhazraei: Review of electrochemical properties of hybrid coating systems on Mg with plasma electrolytic oxidation process as pretreatment, Surf. Interfaces, 14(2019), 262–295, doi: 10.1016/j.surfin.2019.01.004
[31] M. Kaseem; S. Fatimah; N. Nashrah; Y.G. Ko: Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance, Prog. Mater. Sci., 117(2020), 100735, doi: 10.1016/j.pmatsci.2020.100735
[32] M. Molaei; M. Nouri; K. Babaei; A. Fattah-Alhosseini: Improving surface features of PEO coatings on titanium and titanium alloys with zirconia particles: A review. Surf. Interfaces, 22(2021), 100888, doi: 10.1016/j.surfin.2020.100888
[33] Z.Q. Yao; Yu. Ivanisenko; T. Diemant; A. Caron; A. Chuvilin; J.Z. Jiang; R.Z. Valiev; M. Qi; H.-J. Fecht: Synthesis and properties of hydroxyapatite-containing porous titania coating on ultrafine-grained titanium by micro-arc oxidation, Acta Biomater., 6(2010), 2816–2825, doi: 10.1016/j.actbio.2009.12.053
[34] Y. Wang; H. Yu; C. Chen; Z. Zhao: Review of the biocompatibility of micro-arc oxidation coated titanium alloys, Mater. Des., 85(2015), 640–652, doi: 10.1016/j.matdes.2015.07.086
[35] Q.K. Naji Chabuk; J.M. Salman Al-Murshdy; N.M. Dawood: Review: The surface modification of pure titanium by micro-arc oxidation (MAO) process, J. Phys.: Conf. Ser., 1973(2021)1, 012114, doi: 10.1088/1742-6596/1973/1/012114
[36] M. Aliofkhazraei; D.D. Macdonald; E. Matykina; E.V. Parfenov; V.S. Egorkin; J.A. Curran; S.C. Troughton; S.L. Sinebryukhov; S.V. Gnedenkov; T. Lampke; F. Simchen; H.F. Nabavi: Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations, Appl. Surf. Sci. Adv., 5(2021), 100121, doi: 10.1016/j.apsadv.2021.100121
[37] D.-S. Tsai; C.-C. Chou: Review of the soft sparking issues in plasma electrolytic oxidation, Metals, 8(2018)2, 105, doi: 10.3390/met8020105
[38] P. Gupta; G. Tenhundfeld; E.O. Daigle; D. Ryabkov: Electrolytic plasma technology: Science and engineering - An overview, Surf. Coat. Technol., 201(2007), 8746–8760, doi: 10.1016/j.surfcoat.2006.11.023
[39] Q. Chen; W. Li; K. Ling; R. Yang: Investigation of growth mechanism of plasma electrolytic oxidation coating on Al-Ti double layer composite plate, Materials, 12(2019)2, 272, doi: 10.3390/ma12020272
[40] Y. Sasikumar; K. Indira; N. Rajendran: Surface modification methods for titanium and its alloys and their corrosion behavior in biological environment: A review, J. Bio. Tribo. Corros., 5(2019)2, 36, doi: 10.1007/s40735-019-0229-5
[41] Ni, Ao; Liu, D.; Zhang, X.; Guangyu, He: Microstructural characteristics of PEO coating: Effect of surface nanocrystallization, J. Alloys Compds., 823(2020), 153823, doi: 10.1016/j.jallcom.2020.153823
[42] F. Simchen; M. Sieber; A. Kopp; T. Lampke: Introduction to plasma electrolytic oxidation - An overview of the process and applications, Coatings, 10(2020)7, 628, doi: 10.3390/coatings10070628
[43] K. Babaei; A. Fattah-Alhosseini; M. Molaei: The effects of carbon-based additives on corrosion and wear properties of plasma electrolytic oxidation (PEO) coatings applied on aluminum and its alloys: A review, Surf. Interfaces, 21(2020), 100677, doi: 10.1016/j.surfin.2020.100677
[44] J.A. Curran; H. Kalkanci; Yu Magurova; T.W. Clyne: Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications, Surf. Coat. Technol., Spl. Issue, 201(2007)21, 8683–8687, doi: 10.1016/j.surfcoat.2006.06.050
[45] Q. Xia; J. Wang; G. Liu; H. Wei; D. Li; Z. Yao; Z. Jiang: Effects of electric parameters on structure and thermal control property of PEO ceramic coatings on Ti alloys, Surf. Coat. Technol. C, 307(2016), 1284–1290, doi: 10.1016/j.surfcoat.2016.07.073
[46] R.O. Hussein; D.O. Northwood: Chapter 11, Production of Anti-Corrosion Coatings on Light Alloys (Al, Mg, Ti) by Plasma-Electrolytic Oxidation (PEO) in Developments in Corrosion Protection, M. Aliofkhazraei (Editor), IntechOpen, (2014), 201–239, doi: 10.5772/57171
[47] V.M. Frauchiger; F. Schlottig; B. Gasser; M. Textor: Anodic plasma-chemical treatment of CP titanium surfaces for biomedical application, Biomaterials, vol. 25(2004)4, 593–606, doi: 10.1016/s0142-9612(03)00560-x
[48] M-T. Tsai; Y-Y. Chang; H-L. Huang; Y-H. Wu; T-M. Shieh: Micro-arc oxidation treatment enhanced the biological performance of human osteosarcoma cell line and human skin fibroblasts cultured on titanium-zirconium films, Surf. Coat. Technol., A, 303(2016), 268–276, doi: 10.1016/j.surfcoat.2016.03.001
[49] S. Yu; Z. Yu; D. Guo; H. Zhu; M. Zhang; J. Han; Z. Yu; Y. Cao; G. Wang: Enhanced bioactivity and interfacial bonding strength of Ti3Zr2Sn3Mo25Nb alloy through graded porosity and surface bioactivation, J. Mater. Sci. Technol., 100(2022), 137–149, doi: 10.1016/j.jmst.2021.06.008
[50] L. Xu; K. Zhang; C. Wu; X. Lei; J. Ding; X. Shi; C. Liu: Micro-arc oxidation enhances the blood compatibility of ultrafine-grained pure titanium, Materials, 10(2017)12, 1446, doi: 10.3390/ma10121446
[51] L. Liang; Qi. Huang; H. Wu; H. He; G. Lei; D. Zhao; K. Zhou: Engineering nano-structures with controllable dimensional features on micro-topographical titanium surfaces to modulate the activation degree of M1 macrophages and their osteogenic potential, J. Mater. Sci. Technol., 96(2022), 167–178, doi: 10.1016/j.jmst.2021.03.078
[52] Y. Li; W. Wang; J. Duan; M. Qi: A super-hydrophilic coating with a macro/micro/nano triple hierarchical structure on titanium by two-step micro-arc oxidation treatment for biomedical applications, Surf. Coat. Technol., 311(2017), 1–9, doi: 10.1016/j.surfcoat.2016.12.065
[53] S. Sridhar; A. Viswanathan; K. Venkateswarlu; N. Rameshbabu; N. Parthasarathi: Enhanced visible light photocatalytic activity of P-block elements (C, N and F) doped porous TiO2 coatings on Cp-Ti by micro-arc oxidation, J. Porous Mater., 22(2015)2; 545–557, doi: 10.1007/s10934-015-9925-9
[54] A. Sharma; Y.J. Jang; J.P. Jung: Effect of KOH to Na2SiO3 ratio on microstructure and hardness of plasma electrolytic oxidation coatings on AA 6061 alloy, J. Mater. Eng. Perform., 26(2017)10, 5032–5042, doi: 10.1007/s11665-017-2916-z
[55] W. Gebarowski; S. Pietrzyk: Influence of the cathodic pulse on the formation and morphology of oxide coatings on aluminum produced by plasma electrolytic oxidation, Arch. Metall. Mater., 58(2013)1, 241–245, doi: 10.2478/v10172-012-0180-7
[56] E. Erfanifar; M. Aliofkhazraei; H.F. Nabavi; A.S. Roughaghdam: Growth kinetics and morphology of microarc oxidation coating on titanium, Surf. Coat. Technol., 315(2017), 567–576, doi: 10.1016/j.surfcoat.2017.03.002
[57] G. Sundararajan; L.R. Krishna: Mechanisms underlying the formation of thick alumina coatings through the MAO coating technology, Surf. Coat. Technol., 167(2003)2–3, 269–277, doi: 10.1016/S0257-8972(02)00918-0
[58] J. Curran; T.W. Clyne: Thermo-physical properties of plasma electrolytic oxide coatings on aluminium, Surf. Coat. Technol., 199(2005)2–3, 168–176, doi: 10.1016/j.surfcoat.2004.09.037
[59] C.S.Dunleavy; I.O. Golosnoy; J. Curran; T.W. Clyne: Characterisation of discharge events during plasma electrolytic oxidation, Surf. Coat. Technol., 203(2009)22, 3410–3419, doi: 10.1016/j.surfcoat.2009.05.004
[60] J. Curran; T. Clyne: The thermal conductivity of plasma electrolytic oxide coatings on aluminium and magnesium, Surf. Coat. Technol., 199(2005) 177–183, doi: 10.1016/j.surfcoat.2004.11.045
[61] Q. Li; J. Liang; Q. Wang: Chapter 4, Plasma Electrolytic Oxidation Coatings on Lightweight Metals, in Modern Surface Engineering Treatments, M. Aliofkhazraei (Editor), IntechOpen, London, (2013), 75–99, doi: 10.5772/55688
[62] Q. Li; W. Yang; C. Liu; D. Wang; J. Liang: Correlations between the growth mechanism and properties of micro-arc oxidation coatings on titanium alloy: Effects of electrolytes, Surf. Coat. Technol., 316(2017), 162–170, doi: 10.1016/j.surfcoat.2017.03.021
[63] S. Aliasghari; P. Skeldon; G. Thompson: Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci., 316(2014)1, 463–476, doi: 10.1016/j.apsusc.2014.08.037
[64] X.-J. Li; G-A. Cheng; W.-B. Xue; R.-T. Zheng; Y.-J. Chengc: Wear and corrosion resistant coatings formed by microarc oxidation on TiAl alloy, Mater. Chem. Phys., 107(2008), 148–152, doi: 10.1016/j.matchemphys.2007.06.067
[65] L. Zhu; J. Qiu; J. Chen; W. Zhang; Z. Chen; T. Zhang; F. Wang: Microstructure and corrosion resistance of the PEO coating on extruded Al6Cu alloy, Surf. Coat. Technol., 369(2019), 116–126, doi: 10.1016/j.surfcoat.2019.04.027
[66] R.O. Hussein; D.O. Northwood; X. Nie: Processing-microstructure relationships in the plasma electrolytic oxidation (PEO) coating of a magnesium alloy, Mater. Sci. Appl., 5(2014)3, 124–139, doi: 10.4236/msa.2014.53017
[67] V. Saenz de Viteri; E. Fuentes: Chapter 5, Titanium and Titanium Alloys as Biomaterials, in Tribology-Fundamentals and Advancements, J. Gegner (Editor) IntechOpen, (2013), 154–181, doi: 10.5772/55860
[68] J.A. Curran; T.W. Clyne: Porosity in plasma electrolytic oxide coatings, Acta Materialia, 54(2006), 1985–1993, doi: 10.1016/j.actamat.2005.12.029
[69] Y. Han; J. Sun; X. Huang: Formation mechanism of HA-based coatings by micro-arc oxidation. Electrochem. Commun., 10(2008)4, 510–513, doi: 10.1016/j.elecom.2008.01.026
[70] A. Krzakala; A. Kazek-Kesik; W. Simka: Application of plasma electrolytic oxidation to bioactive surface formation on titanium and its alloys, RSC Adv., (2013)3, 19725, doi: 10.1039/c3ra43465f
[71] A. Kurup; P. Dhatrak; N. Khasnis: Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review, Materials Today: Proc., 39(2021)1, 84–90, doi: 10.1016/j.matpr.2020.06.163
[72] P. Pesode; S. Barve: Surface modification of titanium and titanium alloy by plasma electrolytic oxidation process for biomedical applications: A review, Materials Today: Proc., 46(2021)1, 594–602, doi: 10.1016/j.matpr.2020.11.294
[73] A.R. Ribeiro, F. Oliveira, L.C. Boldrini, P.E. Leite, P. Falagan-Lotsch, A.B.R. Linhares, W.F. Zambuzzi, B. Fragneaud, A.P.C. Campos, C.P. Gouvêa, B.S. Archanjo, C.A. Achete E. Marcantonio Jr., L.A. Rocha and J.M. Granjeiro: Micro-arc oxidation as a tool to develop multifunctional calcium-rich surfaces for dental implant applications, Mater. Sci. Eng., 54(2015), 196–206, doi: 10.1016/j.msec.2015.05.012
[74] Z. Yao; Q. Shen; A. Niu; B. Hu; Z. Jiang: Preparation of high emissivity and low absorbance thermal control coatings on Ti alloys by plasma electrolytic oxidation, Surf. Coat. Technol., 242(2014), 146–151, doi: 10.1016/j.surfcoat.2014.01.034
[75] D. Madhuri; R. Ghosh; M.A. Hasan; A. Dey; A.M. Pillai; K.S. Anantharaju; A. Rajendra: Development and characterization of high emittance and low-thickness plasma electrolytic oxidation coating on Ti6Al4V for spacecraft application, J. Mater. Eng. Perform., 30(2021)6, 4072–4082, doi: 10.1007/s11665-021-05862-6



![Fig. 2: Plasma electrolytic oxidation process set-up [53] gt 2023 09 074](/images/stories/Abo-2023-09/thumbnails/thumb_gt-2023-09-074.jpg)