Due to the discovery of unique physical and chemical properties of cobalt-based alloys, their study is of scientific and practical interest [1–4]. Usually, amorphous structures are obtained by rather complicated and expensive methods such as ultrahigh vacuum sputtering, molecular beam epitaxy, grinding in high-energy ball mills, or relatively cheaper methods such as electrodeposition [5–8]. Currently, there is growing interest in the method of non-stationary alloy deposition, which is due not only to its efficiency and relative simplicity, but also due to the wide possibilities of controlling the crystallisation kinetics and, consequently, changing the physicochemical properties of electrodeposited alloys [9–11].
As a rule, the amorphous state in Co films is achieved by introducing a second component. It should be noted that electrodeposition is usually carried out at direct current and the phase composition of the resulting alloy depends on the percentage of the amorphizing additive in the electrolyte. However, in pulse current electrodeposition, it is possible to influence the phase composition by varying the pulse current parameters. It should be noted that varying the frequency and duty cycle of the current pulses makes it possible to fully reveal all the advantages of using non-stationary electrolysis compared to stationary one [12–14]. This work is devoted to the study of the influence of pulse electrolysis on the phase composition and properties of Co-W films.
Materials and Methods
Co-W alloy films were obtained by electrodeposition from ammonia electrolytes of the following composition (g/L): CoSO4 – 10, C6H8O7 – 60, Na2WO4 – 6÷16. The pH = 11 was achieved by adding aqueous ammonia. The electrolyte temperature was maintained constant and equal to 333 K. The electrodeposition process is schematically depicted in Figure 1.
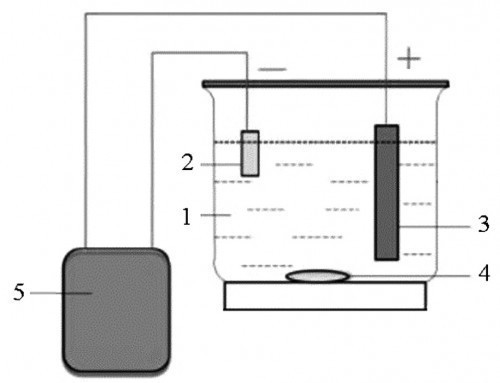 Fig. 1: Schematic diagram showing different components of the electrolytic cell: 1 – electrolytic bath; 2 – cathode; 3 – anode; 4 – stirrer; 5 – pulse generator (all figures and tables: Valentina Tytarenko)
Fig. 1: Schematic diagram showing different components of the electrolytic cell: 1 – electrolytic bath; 2 – cathode; 3 – anode; 4 – stirrer; 5 – pulse generator (all figures and tables: Valentina Tytarenko)
Plates of pure cobalt were used as an anode for electrodeposition. This allowed to keep the concentration of crystallising metal ions constant, which positively influenced the repeatability of experiments. Copper foil was used as a substrate during electrodeposition. The foil for the substrates was prepared as follows. First, the substrates were mechanically and chemically polished. The solution for chemical polishing was 5 % nitric acid solution. Chemical polishing reduced the roughness and removed the work hardening formed after mechanical polishing. Then the substrates were degreased in the Vienna lime solution and washed in distilled water.
Electrodeposition was carried out by rectangular current pulses. The current pulse repetition frequency (f) varied from 20 Hz to 300 Hz. The duty cycle (Q) varied from 2 to 6. The average current density remained constant and was equal to 6 A/dm2. The average pulse current density was chosen so that the forming film had a qualitative appearance.
Studies of the phase composition of Co-W films were carried out on an X-ray diffractometer DRON-2 using a scintillation registration of X-rays. Imaging for the phase composition of the films was carried out in monochromatized Co-Kα radiation. The elemental composition was determined on X-ray spectrometers VRA 20, VRA 30 by measuring the intensity of analytical Kα lines for iron elements (35 kV, W - anode), and for phosphorus (35 kV, Rh – anode). The microhardness of the films was measured on a microhardness tester PMT-3 with an indenter load of 0,2 N.
Results and their discussion
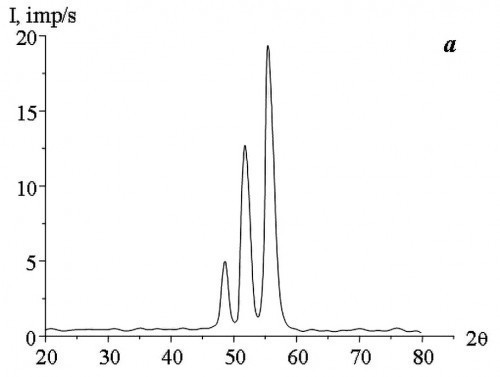
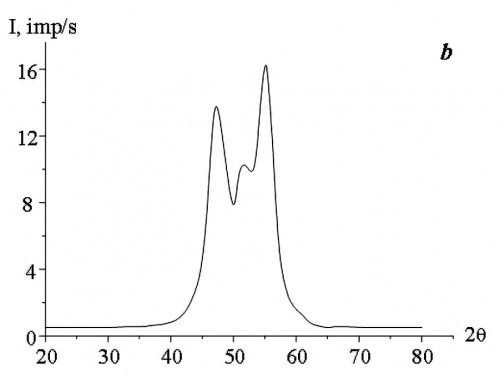
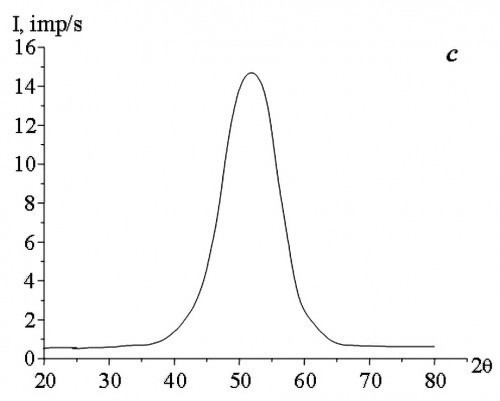
The study of the phase composition of Co-W alloys obtained by electrodeposition at direct and pulse currents showed that, depending on the concentration of sodium tungstate salts (Na2WO4) in the aqueous electrolyte solution, the alloys have an amorphous, amorphous-crystalline or crystalline structure (Fig. 2).
According to the results of X-ray diffraction analysis, the cobalt and tungsten atoms did not combine into separate phases (Co3W and Co7W6).
In the films obtained by DC electrodeposition, the X-ray amorphous state was observed at a concentration of Na2WO4 in the aqueous electrolyte solution of not less than 11 g/L. Application of pulse current allowed to obtain X-ray amorphous Co-W alloy at a lower concentration of Na2WO4 in the aqueous electrolyte solution. The study found the minimum permissible values of Na2WO4 concentration in the aqueous electrolyte solution, at which X-ray amorphous alloys are obtained as a result of pulse electrodeposition, with an increase in „severity“ of the deposition modes, i.e. with increasing the duty cycle (Q) and decreasing the current pulse repetition frequency (f).
|
Q |
f, Hz |
||
|
20 |
200 |
400 |
|
|
2 |
C |
C |
C |
|
4 |
А+C |
А+C |
C |
|
6 |
А |
А+C |
А+C |
Table 1 shows the results of studying the influence of pulsed electrodeposition modes on the phase composition of Co-W films obtained from the aqueous electrolyte solution with Na2WO4 content of 8 g/L. The table shows that using pulse electrodeposition modes with parameters f=20 Hz and Q=6 it was possible to obtain amorphous Co-W alloy from the aqueous electrolyte solution containing Na2WO4 with a concentration of 8 g/L, while using direct current the amorphous state of the alloy was achieved at Na2WO4 concentration of not less than 11 g/L. Thus, it was found that during pulsed electrodeposition, the formation of an amorphous phase is affected not only by the concentration of the amorphizing substance (Na2WO4), but also by the deposition modes (current pulse repetition frequency and pulse duty cycle).
The results of the studies (Tab. 1) showed that with increasing f and decreasing Q the structure of Co-W alloys becomes more equilibrium, under unchanged deposition conditions (composition and temperature of the aqueous electrolyte solution, average current density). The change in crystallization conditions can be explained by the fact that during pulsed electrolysis, with an increase in the repetition frequency of current pulses and a decrease in their duty cycle, the supersaturation value at the crystallization front decreases with decreasing rate of change of cathodic supersaturation [15], i.e., the conditions necessary for the formation of an amorphous state are not reached. The formation of two-phase films (amorphous and crystalline phases) is caused by the fact that during the pause between current pulses, the cathodic supersaturation drops to a certain residual value that is not equal to zero. During the action of the current pulse in the cathode region, supersaturation is reached, which is sufficient for the introduction of the required amount of tungsten for the formation of an amorphous state. The pause between current pulses is characterized by a low rate of change in cathode supersaturation, which, to a large approximation, can be considered as areas of direct current. At the same time, the value of cathode supersaturation is much lower than in the pulse. It should be noted that the use of large duty cycles of current pulses at low frequencies leads to an increase in the pause duration, which in some cases can have a noticeable effect on the formation of the structure of electrolytic Co-W alloys (Fig. 3).
|
Deposition mode |
Concentration of |
Content W in the alloy, mas. % |
Microhardness, МPа |
|
DC |
16 |
36 |
5100-5400 |
|
14 |
34,5 |
4900-5200 |
|
|
12 |
33 |
4700-5000 |
|
|
16 |
35 |
5300-5600 |
|
|
Pulse current |
14 |
33,8 |
5000-5300 |
|
12 |
32,2 |
4800-5100 |
Table 2 shows the results of spectral analysis and microhardness study of Co-W alloys obtained by electrodeposition from electrolyte solutions with different sodium tungstate content using direct and pulse currents. The table shows that with increasing concentration of sodium tungstate in the aqueous electrolyte solution and, accordingly, the tungsten content in the alloy, there is an increase in the values of microhardness of the electrodeposited films. Comparing the values of microhardness of the alloys obtained by means of direct and pulse currents, we conclude that the use of pulse modes of deposition allows to obtain Co-W alloys with higher microhardness. It should also be noted that the alloys obtained using pulse current contain less tungsten compared to alloys obtained using direct current, other conditions of deposition being equal (composition and temperature of the aqueous electrolyte solution, average current density).
This is due to the fact that during pulsed deposition, as a result of the high overpotential at the cathode, there is a strong release of hydrogen in the cathode region, which prevents the incorporation of tungsten into the coating.
Conclusions
The use of pulse current in the electrodeposition of amorphous Co-W alloys, in comparison with electrodeposition using direct current, made it possible to:
- reduce the required concentration of the amorphizing additive (sodium tungstate) in the aqueous electrolyte solution by 27 %
- obtain from one electrolyte solution the alloys in both amorphous and crystalline states, which in turn makes it possible to obtain layered structures consisting of alternating layers of the amorphous and crystalline phases
- increase the microhardness of electrodeposited films from 4800 MPa to 5600 MPa.
References:
[1] Yu Yundan et al.: Surf. Rev. Lett., 16 (4) (2009) 635–642, https://doi.org/10.1142/S0218625X09012950
[2] Anuj Kumar et al.: Mater. Res. Express, 1 (3) (2014) 035007, https://doi.org/10.1088/2053-1591/1/3/035007
[3] Ma Liwen et al.: Int. J. Electrochem. Sci., 12 (2) (2017) 1034–1051, https://doi.org/10.20964/2017.02.37
[4] Mrinalini Mulukutla et al.: Applied Surface Science, 258 (7) (2012) 2886–2893, https://doi.org/10.1016/j.apsusc.2011.11.002
[5] Gulmira Yar-Mukhamedova et al.: Applied Surface Science, 445 (2018) 298–307, https://doi.org/10.1016/j.apsusc.2018.03.171
[6] D.P. Weston et al.: Transactions of the IMF. 88 (1) (2010) 47–56, https://doi.org/10.1179/174591909X12596810686490
[7] Y.S. Yapontseva et al.: Surf. Engin. Appl.Electrochem., 50 (2014) 330–336, https://doi.org/10.3103/S1068375514040139
[8] V.V. Shtefan et al.: Mater Sci, 43 (2007) 429–433, https://doi.org/10.1007/s11003-007-0049-5
[9] Fenghua Su et al.: Wear, 300 (1-2) (2013) 114–125, https://doi.org/10.1016/j.wear.2013.01.120
[10] Somayeh Abazari et al.: Journal of Materials Engineering and Performance, (26) (2017)3133–3143, https://doi.org/10.1007/s11665-017-2698-3
[11] J.M. Costa et al.: Chem. Pap., 73 (2019) 1103–1112, https://doi.org/10.1007/s11696-018-0661-x
[12] V.V. Tytarenko et al.: Inorganic materials: applied research, 10 (3) (2019) 589–59, https://doi.org/10.1134/S2075113319030419
[13] V.V. Tytarenko et al.: Metallofizika i Noveishie Tekhnologiithis, 42(3) (2020) 351–362, https://doi.org/10.15407/mfint.42.03.0333
[14] V.V. Tytarenko et al.: Galvanotechnikthis, 110(4) (2019) 648–651, http://eadnurt.diit.edu.ua/jspui/bitstream/123456789/11211/1/Tytarenko.pdf
[15] V.A. Zabludovsky et al.: Programmnyj impulsnyj elektroliz metallov i kompozicionnyh materialov [Program pulsed electrolysis of metals and composite materials], Lambert Academic Publishing, Saarbrücken (2019) 250, http://eadnurt.diit.edu.ua/jspui/bitstream/123456789/11210/1/Zabludovsky.pdf