Factors Influencing PEO Process Substrate Materials
PEO technique is a conversion coating process; hence the substrate material plays a crucial role in determining the composition and properties of resultant oxide coating. Further, the different alloys will have different breakdown potentials, the discharge characteristics and voltage time response may vary accordingly. Figure 1 shows the different application sectors of Plasma Electrolytic Oxidation.
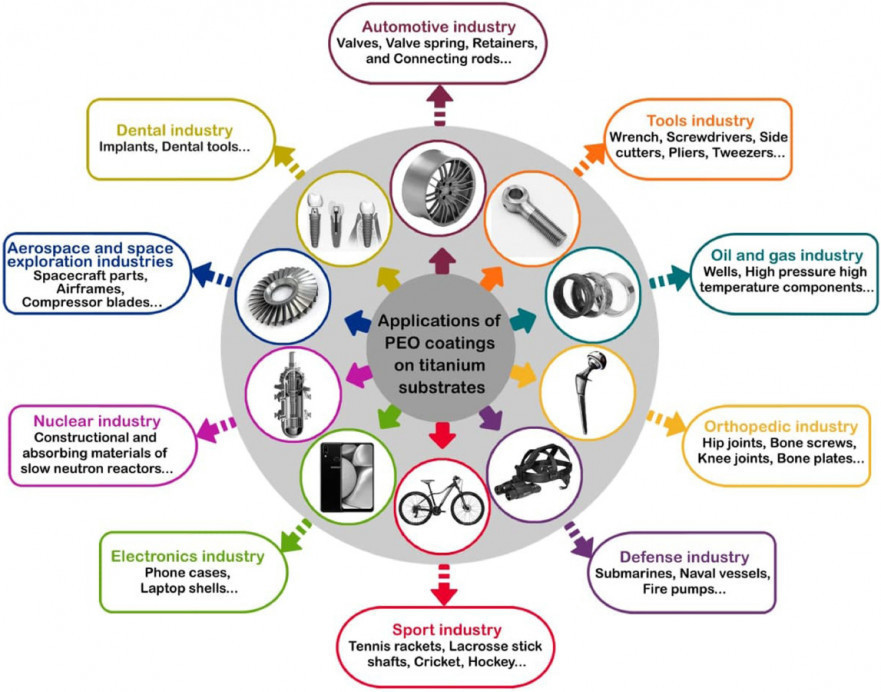 Fig. 1: Titanium substrates are coated by Plasma Electrolytical Oxidation technology in a broad variety of production sectors [45]
Fig. 1: Titanium substrates are coated by Plasma Electrolytical Oxidation technology in a broad variety of production sectors [45]
Wang et al. [1] investigated the influence of alloy elements in pure titanium, Ti6Al4V and Ti35Nb2Ta3Zr on the structure, morphology and properties of PEO coatings in sodium silicate electrolyte. The Ti35Nb2Ta3Zr alloy has better film-forming properties – a nested membrane with a porous structure was formed. The relative content of anatase phase with biological activity is much more than that of the rutile phase as compare to pure Ti and Ti6Al4V, which can effectively improve the deposition capacity of apatite. Furthermore, the PEO treated Ti35Nb2Ta3Zr has excellent corrosion resistance due to its denser and thicker coating, as well as the formation of the passivation film owing to the existence of Nb2O5 phase on the surface. The wetting angle test showed that the coating on Ti35Nb2Ta3Zr has long-lasting and excellent hydrophilic properties.
A comparison of tribocorrosion and electrochemical impedance of PEO coating on pure Ti and Ti6Al4V alloy was reported by Fazel and team [2]. The PEO coatings were obtained 180 V. Unlike the volcanic morphology of PEO/Ti, PEO/Ti6Al4V showed many vermiform slots. Electrochemical Impedance Spectroscopy (EIS) analysis of PEO/Ti specimens showed a lower capacitance of the barrier layer; fretting fatigue cracks propagate and cause coating delamination. Conversely, no delamination was detected in PEO/Ti6Al4V and a mild abrasive wear was the dominant mechanism in tribocorrosion.
Nature of Electrolyte
In addition to substrate metal oxide as the main component, PEO coating as well incorporates some complex oxides and compounds due to reaction with electrolyte. The coating growth rate, composition, morphology, microstructure and resultant properties are also influenced by the electrolyte composition. The PEO process is mostly
accomplished in the mild alkaline electrolytes consisting of dilute solutions of sodium or potassium hydroxides, silicates, phosphates or aluminates or mixtures of these. KOH or NaOH are usually added to the electrolyte to increase the electrolyte conductivity and to stabilize the discharges [3]. When KOH is added to the electrolyte, the breakdown voltage is reduced, pores size becomes smaller [4], and porosity is slightly decreased [5].
Acidic solutions of sulfuric and phosphoric acids promote different morphologies based on the applied regime, acid concentration, and substrate. Coating in phosphoric acid with low concentration leads to the formation of porous microstructure with rounded pores while increasing acid concentration promotes the formation of grooved shaped pores [6]. Grooves or craters are developed due to the sharp increase of the pressure in the micro spaces. These grooved morphology coatings have a higher roughness compared to the microstructures with rounded pores [7].
Yerokhin et al. [8] investigated PEO coatings on Ti6Al4V alloy with different compositions of electrolyte under constant anodic power density. The results have shown that the coatings produced in an aluminate-phosphate electrolyte provided the highest wear resistance, although with a relatively high coefficient of friction. These coatings contained TiAl2O5 and rutile. The coatings are relatively dense and uniform, whereas coatings formed in a silicate electrolyte are porous and contain amorphous and crystalline silica and Ti3O5. The surface characteristics of pure titanium subjected to a plasma electrolytic oxidation using two different electrolytes containing either potassium pyrophosphate or potassium triphosphate were investigated by Shin et al. [9]. PEO coatings were fabricated at ambient temperature with a current density of 200 mA/cm2. The volume fraction of metastable anatase phase in the PEO coating formed with potassium pyrophosphate was relatively higher than that with potassium triphosphate. The film formed with potassium pyrophosphate electrolyte showed the easy formation of biomimetic apatite in a simulated body fluid solution.
Effects of electrolytic concentration on bioactive PEO films containing Ca and P on the surface of the Ti6Al4V alloy was studied by Shi and co-investigators [10]. With an increase in electrolytic concentration, the ratio of rutile in films increased and small amounts of calcium phosphate and hydroxyapatite appeared. As the microporosity and surface roughness of film increases with concentration, the wettability of the oxide film improves continually, while microhardness increases at first and then decreases. The PEO coatings on CPTi were formed in different electrolytes compositions (aluminate, phosphate, silicate, aluminate-phosphate, and aluminate-silicate), and the influence on the discharge characteristics, macro- and micro-structure, thickness, phase compositions, and corrosion behaviour of coatings was evaluated [11]. The PEO processes were carried out at fixed voltage (420 V) for 180 seconds. The most uniform and dense structure PEO coatings with least porosity and higher corrosion resistance were obtained in aluminate-phosphate-based electrolyte. These coating consisted of TiO2 (rutile, anatase) and TiAl2O5 phases.
In a study Ping et al. [12] reported that the concentration of NaAlO2 effects the characteristics of the PEO coatings on pure titanium. With the increase in NaAlO2 concentration, the working voltage increases that promotes the growth rate of the coatings. The number and size of the sintered disc on PEO coatings increased gradually while the number of micro-pores decreased. The content of the TiO2 phase decreases as NaAlO2 increases. The influence of electrolyte composition on micro- structure and properties of PEO coatings on pure Ti substrate was examined by Yang and co-workers [13]. Three PEO coatings were prepared in the Na2SiO3 base solution and with K2ZrF6 or K2TiF6 addition. PEO coating formed in electrolyte with K2ZrF6 or K2TiF6 additives exhibited improved hardness and a self-sealing microstructure. While K2TiF6 addition resulted into low arc starting voltage which led to increase in growth rate, and the coating formed also exhibited the superior corrosion resistance.
Simchen et al. [14] used Na2SiO3, KOH and NaAlO2 solutions to investigate the influence of electrolyte conductivity, concentration and anionic species on the ignition voltage of Al, Mg and Ti alloys in the PEO process. It was demonstrated that not the electrical electrolyte conductivity, but its specific electron injection ability at the electrolyte/substrate interface defines the ignition voltage. This injection ability depends on the kind and concentration of the electrolytic anions.
Additives
Inclusion of different additives in the electrolyte can greatly affect the PEO process, influencing the properties of the coatings. Although inorganic electrolytes are widely used in PEO, of late the organic additives have received considerable interest. The organic additives tend to adsorb on the surface of metallic substrates during the PEO process through their functional groups (−OH, –COOH, –NH–CO–, etc.). This prevents the inorganic additives – the main components of the electrolyte and dissolved oxygen from transferring to the anodic substrate, resulting in easy increments in the cell voltage and controlling spark discharging. Among different organic acids the EDTA is a most promising additive due to its ability to form a relatively stable complex with metallic ions. The progress of organic additives on the electrical response and the plasma discharges behaviour during the PEO process is summarized by Kaseem et al. [15].
The porous gray-black coatings were formed when oxides of various valence states of some transition elements (Fe, Co, Ni, Ti, V and W) were incorporated into the coating [4,16]. A process of flat absorber PEO coating on Ti6Al4V alloy in phosphate electrolyte containing sodium tungstate was investigated by Yao et al. [17]. The top-notch results were obtained with Na5P3O10: 70 g/L, EDTA-2Na: 30 g/L, FeSO4: 15 g/L, Co(CH3COO)2: 15 g/L, Ni(CH3COO)2: 15 g/L and Na2WO4: 7.5 g/L. A pulsed current density of 150 A/dm2 was employed in a constant current regime with a working frequency of 1000 Hz for 25 minutes. The reaction temperature was controlled below 30 °C. Under these conditions, a solar absorptance of 0.93 and thermal emittance of 0.88 was achieved with a coating thickness of ~40 µm and a roughness of ~1.3 µm.
A black PEO coating with high absorptivity (0.962) and emissivity (0.950) was prepared on TA7 (Ti5Al2.5Sn) alloy in a hybrid electrolyte containing 3 g/L NH4VO3, 5 g/L FeSO4, and 5 g/L C4H6O4Ni under 400 V for 10 minutes [18]. The coating with typically porous structure is mainly composed of O, P, Si, Ti, V, Fe, and Ni. The corresponding amorphous oxide in the outer layer endows the coating with strong absorption in the visible light and infrared areas, and the crystallized TiO2 indwelling the inner layer contributes to the strong UV absorption property. In addition, the micropores of the coatings have different size ranges corresponding to the wavelengths, facilitating the increase of absorptivity and emissivity to some degree.
Black PEO coating on Ti6Al4V alloy was prepared with the colouring additives of NH4VO3, FeSO4 and Co(CH3COO)2 [19]. The coating had high absorption (> 0.95, 200–2500 nm) and emission (> 0.92, 2.5–16 μm) when the reaction time was over 30 minutes. The high absorption and emission were associated with d-d transition, and infrared stretching vibration of transition metal oxides, as well as complex porous structure on the surface. These coatings showed excellent thermal stability in air up to 300 °C or oxygen deficient environment up to 500 °C.
Tb doped TiO2 coatings on titanium were formed in aqueous solution of 10 g/L Na3PO4·12H2O with addition of Tb4O7 powder [20], and their photocatalytic activity (PA) was examined under simulated sunlight conditions. Tb3+ doped coatings formed after 1 minute of PEO process have better PA than pure TiO2 coatings formed under the same conditions. Presence of Tb3+ ions into TiO2 coatings in small concentration improves PA through effective suppression of electron/hole recombination.
Electrical Parameters
The phase composition and the properties of PEO coatings are greatly affected by electrical parameters, viz, current modes, current density, current frequency, anodic voltage, cathodic voltage, duty cycle, etc. Huang et al. [21] investigated the effects of bath voltage on mechanical properties of PEO coating on Ti. The treatment was carried out in an aqueous solution containing calcium and phosphate salts, using different voltages from 240 V to 450 V. The composition of the PEO coatings was generally anatase and rutile, while at higher voltage of 400-450 V, a new CaTiO3 phase appeared. The pore size, elastic modulus and residual stress of coating increased with the rise of applied voltage. The bonding strength of layer was above 30 MPa. The samples prepared at 240-350 V had much stronger bonding strength compared to that prepared at higher voltage.
The effects of cathode voltage on structure and properties of MAO coatings on equiatomic biomedical NiTi alloy were investigated by Liu et al. [22]. The MAO processes were carried in a solution of sodium aluminate (0.15 M) and sodium hypophosphite (0.01 M) using a pulsed bipolar power supply. The anodic voltage was kept constant at 400 V while the cathodic voltage was controlled at 0, 10, 20, and 30 V, respectively. The MAO coatings were consisting of γ-Al2O3 as the only crystalline phase, and the crystallinity can be enhanced by increasing the cathodic voltages. The thickness and surface roughness of the coatings increased with increasing cathodic voltage. The Ni concentration on the surface layer increases with increasing cathodic voltage. The friction coefficient of the coatings increased, while the bonding strength, corrosion resistance and biocompatibility reduced with increasing cathodic voltage.
Different morphologies can be obtained by changing the applied voltage regimes. PEO coatings obtained on CPTi Grade 2 in electrolyte containing 500 g of Ca(NO3)2· 4H2O or Mg(NO3)2–6H2O in 1 dm3 of concentrated (85 wt%) H3PO4 for 3 minutes using DC and AC voltages under controlled conditions by Rokosz et. al. [23]. The DC process was performed under constant voltage of 450 V while the AC process was conducted with sinusoidal shape of voltage control, using peak-to-peak voltage of 200 Vpp at frequency of 50 Hz. A coarser morphology coating is obtained by using 450 V DC in comparison with 200 Vpp AC when Ca or Mg enriched coatings are produced.
PEO coatings in an electrolyte containing CaOH and Na3PO4·12H2O were deposited on the β-type low elastic modulus Ti29Nb13Ta4.6Zr (TNTZ) alloys to enhance the in-vitro corrosion resistance [24]. The effect of duty cycle, deposition frequency, coating thickness, and surface morphologies have been evaluated. The corrosion rates of the coated samples were approximately 4-14 times lower than the uncoated sample. The highest corrosion resistant coating was obtained at 500 Hz with 30 % duty cycle. Influence of duty cycle and frequency on DC MAO of CP-Ti was investigated by Gowtham et al. [25]. The coating produced at higher duty cycle (95 %) and higher frequency (1000 Hz) exhibited the formation of denser coating with a higher thickness (~ 9 µm), which provides better corrosion and scratch resistance. These coatings were relatively less porous due to the availability of a short time period for each cycle.
Wear resistant of PEO coatings on CPTi Grade 4 alloys formed in an aluminate and zirconia containing electrolyte (NaOH: 2 g/L, NaAlO2: 40 g/L, m-ZrO2: 4 g/L; pH:13; conductivity: 47 mS/cm) was evaluated [26]. A factorial design of experiments (DoE), by varying the PEO process parameters (current density, repetition rate and duty cycle) was adopted. XRD / GAXRD (glancing angle x-ray diffraction) phase analysis indicated the formation of crystalline aluminium titanate (TiAl2O5), tetragonal zirconia (t-ZrO2) as well as corundum (α-Al2O3), leading to an increase in hardness (15.5 GPa, H/E ≈ 0.08) and wear resistance (6.7×10-5 mm3 N–1m–1). Evaluation of the DoE’s parameter interaction shows that the main effects for generating wear resistant coatings are current density and repetition rate.
Processing Temperature
The processing temperature plays a significant role in any chemical reaction. When the electrolyte temperature is very low, the oxidation process becomes too slow, and if the bath temperature is too high, the dissolution of oxide film is enhanced, which may lead to surface roughness. The MAO process is generally performed at room temperatures. As the coating growth proceeds, the temperature of electrolyte increases, which must be controlled by the cold-water circulation and the bath homogeneity is maintained with suitable agitation of the solution. The effects of different electrolyte temperatures on the wear-resistance of MAO coatings on β-Ti alloy was investigated by Habazaki et al. [27]. The process was carried out in an electrolyte system containing K2Al2O4 (0.15 mol/L), Na3PO4 (0.02 mol/L) and NaOH (0.015 mol/L), at different temperatures between 278 K and 313 K. Results showed that at the lowest temperature of 278 K, the MAO coating is formed with higher concentration of α-Al2O3 phase in addition to the Al2TiO5 as a major phase. The coating exhibited lower porosity, good uniformity and density, and showed improved wear resistance, compared to that formed at higher temperatures.
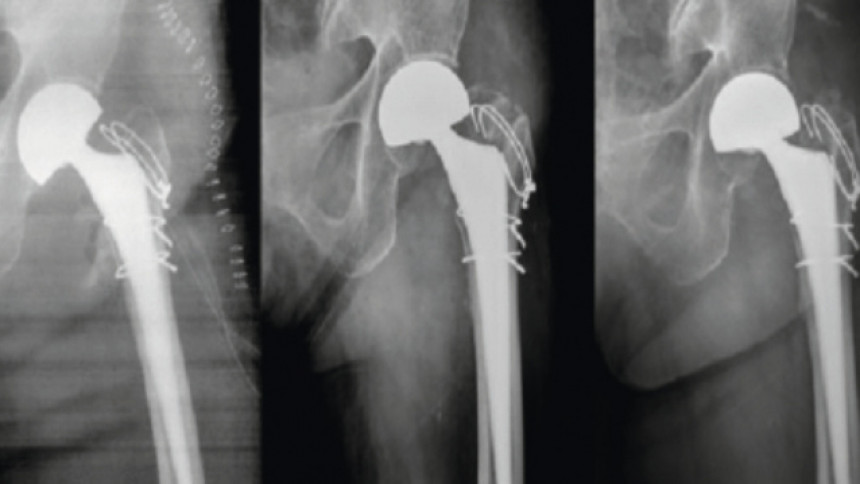
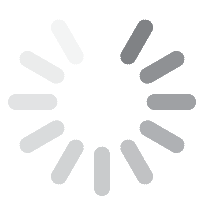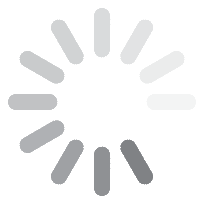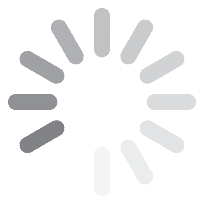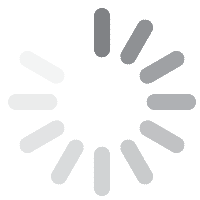
![Fig. 2: MAO coated titanium alloy them and radiographs in body showing a) intertrochanteric fracture; b) immediately after postoperatively; c) After 6 months shows broken wires and gap between the proximal femur and the greater trochanter fragment; d) After 2 years with a stable bone ingrowth [42] gt 2023 10 058](/images/stories/Abo-2023-10/gt-2023-10-058.jpg) Fig. 2: MAO coated titanium alloy them and radiographs in body showing a) intertrochanteric fracture; b) immediately after postoperatively; c) After 6 months shows broken wires and gap between the proximal femur and the greater trochanter fragment; d) After 2 years with a stable bone ingrowth [42]
Fig. 2: MAO coated titanium alloy them and radiographs in body showing a) intertrochanteric fracture; b) immediately after postoperatively; c) After 6 months shows broken wires and gap between the proximal femur and the greater trochanter fragment; d) After 2 years with a stable bone ingrowth [42]
Processing Time
In general, the increase in processing time leads to higher coating thickness and roughness. The influences of MAO time on the microstructures and the distribution of elements in the coating surface with alkaline electrolyte containing Si, Ca and Na elements was investigated [28]. An increase in the contents of Si, Ca, and Na elements on the coating surface was observed with the increasing process time. The pore size is in direct proportion to the processing time, while the numbers of pores are inversely proportional to time.
Composite PEO Coatings Containing Nanoparticles
Attempts were made to produce composite PEO coatings on titanium by in-situ incorporation or sealing of the porous PEO coatings with various particles to improve the microhardness and tribological properties. The coating microstructure and composition can be controlled by particle addition. The nanoparticles are adsorbed to the coating by the electrophoresis force [29, 30]; agglomerate in the pores, and subsequently trapped in the molten substrate oxide as a result of continuous micro-discharging [31, 32]; creating new phases in the oxide layer due to the high temperature of the micro-discharges. For titanium-based alloys, many nanoparticles, such as SiO2 [29], MoS2 [30], Si3N4 [31], Ag [32], ceria [33], B4C [34], etc. have been used to improve the properties and characteristics of PEO coatings [35].
Bio Induction of PEO Coatings
Titanium is widely used in dentistry and orthopaedics metal transplants. If Ca and P ions are incorporated in the electrolyte during the PEO process, the oxide layer produced may contain bioactive hydroxyapatite (HA) material. The HA content can further be increased by subsequent hydrothermal treatment. A state-of-the-art review on production of hydroxyapatite layers on the plasma electrolytically oxidized surface of titanium alloys is presented by Lugovskoy, and Lugovskoy [36]. Karbowniczek et al. [37] synthesized PEO coatings containing Ca and P species on Ti6Al7Nb alloy to improve their biocompatibility and osseointegration. Enhancement in biocompatibility is achieved by the formation of a rough outer layer with open pores that provides high surface area for cells to adhere, proliferate and migrate into the coating.
Liu et al. [38] described the formation of hydroxyapatite on Ti6Al4V alloy by microarc oxidation in the electrolyte containing 0.06 M calcium glycerophosphate and 0.25 M calcium acetate at a current density of 2 A/dm2 (350 V, DC). After hydrothermal treatment, the hydroxyapatite was precipitated (thickness ~ 4 μm) on the surface of the MAO film. Zhang et al. [39] fabricated PEO coatings in a silicate electrolyte containing Na2(EDTA), CaO and Ca(H2PO4)2, using 350 V pulsed DC, a frequency of 200 Hz, a duty cycle of 50 % and a treatment time of 5 minutes. The voltage and time effects on the formation of hydroxyapatite-containing PEO on Ti6Al4V alloy and its corrosion behaviour was studied by Montazeri et al. [40]. The sample coated at 500 V for 15 minutes showed the best corrosion behaviour in Ringer's solution. Effect of time on the formation of hydroxyapatite in PEO process with hydrothermal treatment of the Ti6Al4V Alloy was investigated by Kossenko and his team [41]. Bipolar hemiarthroplasty using microarc oxidation-coated cementless stem in patients with unstable intertrochanteric fracture was investigated by Lee et al. [42]. The results outlined in Figure 2 clearly showed that cementless HA using MAO-coated stem enabled early ambulation in most (76.5 %) of the elderly patients with unstable intertrochanteric fractures. The outcome was very encouraging with a low incidence of venous thromboembolism and stem loosening. The stem remained well fixed with a stable bone ingrowth and the patient could walk independently without any symptom.
Biofunctionalization of the PEO coatings with inorganic components by introducing Ca-, P- containing compounds into the film (primarily hydroxyapatite), or with integrin-active organic components (primarily RGD peptides, RGD = Arginylglycylaspartic acid) was investigated by Parfenov et al. [43]. Titania + RGD seems to be the most promising for the titanium implants. Liao et al. [44] functionalized PEO layer using hexamethyldisilazane (HMDSZ) /O2 plasma treatment at 30 W and 100 mTorr for 5 minutes. The potential polarization dynamic test and Alamar blue cell viability assay showed the improvement in both the corrosion resistance and biocompatibility of surfaces for biomedical applications.
Conclusion
Titanium is an ideal metal for a variety of industrial applications because of its high tensile strength to density ratio, ability to withstand moderately high temperatures, superior corrosion resistance, and osseointegration properties. The major drawback with titanium is its low hardness and wear resistance, and reactivity to atmospheric oxygen. This necessitates a suitable surface modification of titanium before its tangible applications. There is a diversity of pre-treatment procedures that modify the surface morphology of titanium and clear away the unstable oxide layer, and contaminants. Anodizing and plasma electrolytic oxidation have been utilized to synthesize well-controlled corrosion resistant and biocompatible TiO2 films. The substrate composition, and process parameters influence the physical properties of the coating, such as microstructure, pore size, pore roughness, colour, texture, hardness, and surface area. Plasma electrolytic oxidation enables far superior mechanical properties, thermal shock resistance, dielectric strength, and corrosion protection. The PEO coating consists of a porous outer layer, a compact intermediate layer and a thin inner layer. The choice of electrolytes, and operating parameters enables a wider choice of characteristics of the coating. The specific functional properties of oxide coatings can be further improved by the inclusion of suitable additives.
REFERENCES
[1] C.H. Wang; F.C. Ma; P. Liu; X.K. Liu; K. Zhang; Q.Y. Han: The influence of alloy elements in Ti6Al4V and Ti35Nb2Ta3Zr on the structure, morphology and properties of MAO coatings, Vacuum, 157(2018), 229–236, doi: 10.1016/j.vacuum.2018.08.054
[2] M. Fazel; H.R. Salimijazi; M.A. Golozar; M.R. Garsivaz jazi: A comparison of corrosion, tribocorrosion and electrochemical impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process, Appl. Surf. Sci., 324(2015) 751–756, doi: 10.1016/j.apsusc.2014.11.030
[3] M. Asadi; M. Attarchi; M. Vahidifar; A. Jafari: Effect of SiO2-3/OH- on plasma electrolytic oxidation of Ti-5Mo-4V-3Al, Bull. Mater. Sci., 33(2010)4, 469–474, doi: 10.1007/s12034-010-0072-2
[4] I. Han; J.H. Choi; B.H. Zhao; H.K. Baik; I.S. Lee: Micro-arc oxidation in various concentration of KOH and structural change by different cut off potential, Curr. Appl. Phys., 7 (2007), e23–e27, doi: 10.1016/j.cap.2006.11.008
[5] M. Shi; H. Li: A Mathematical interpretation model of Ti alloy micro-arc oxidation (MAO) process and its experimental study, Surf. Eng. Appl. Electrochem., 51(2015)5, 468–477, doi: 10.3103/S1068375515050142
[6] M. Echeverry-Rendon; O. Galvis; D. Quintero Giraldo; J. Pavon; J.L. Lopez-Lacomba; E. Jimenez-Pique; M. Anglada; S.M. Robledo; J.G. Castano; F. Echeverria: Osseointegration improvement by plasma electrolytic oxidation of modified titanium alloys surfaces, J. Mater. Sci. Mater. Med., 26(2015)2, 72, doi: 10.1007/s10856-015-5408-4
[7] M. Shi; H. Li: The morphology, structure and composition of Microarc Oxidation (MAO) ceramic coating in Ca-P electrolyte with complexing agent EDTMPS and interpretation hypothesis of MAO process, Surf. Eng. Appl. Electrochem., 52(2016)1, 32-42. doi: 10.3103/S1068375516010130
[8] A.L. Yerokhin; X. Nie; A. Leyland; A. Matthews; S.J. Dowey: Characterisation of oxide films produced by plasma electrolytic oxidation of a Ti6Al4V alloy, Surf. Coat. Technol., 130(2000), 195–206, doi: 10.1016/S0257-8972(00)00719-2
[9] K.R. Shin; Y.G. Ko; D.H. Shin: Effect of electrolyte on surface properties of pure titanium coated by plasma electrolytic oxidation, J. Alloys Compds., 509(2011), s478–s481, doi: 10.1016/j.jallcom.2011.02.056
[10] X.L. Shi; Q.L. Wang; F.S. Wang; S.R. Ge: Effects of electrolytic concentration on properties of micro-arc film on Ti6Al4V alloy, Mining Sci. Technol., 19(2009), 220–224, doi: 10.1016/S1674-5264(09)60042-9
[11] A. Fattah-Alhosseini; M.K. Keshavarz; M. Molaei; S.O. Gashti: Plasma electrolytic oxidation (PEO) process on commercially pure Ti surface: Effects of electrolyte on the microstructure and corrosion behavior of coatings. Met. Mater. Trans. A, 49(2018), 4966–4979, doi: 10.1007/s11661-018-4824-8
[12] W. Ping; W. Ting; P. Hao; G. X. Yang: Effect of NaAlO2 concentrations on the properties of micro-arc oxidation coatings on pure titanium” Mater. Lett., 170(2016), 171–174, doi: 10.1016/j.matlet.2016.02.024
[13] W. Yang; D. Xu; Q.-Q. Guo; T. Chen: J. Chen, Influence of electrolyte composition on microstructure and properties of coatings formed on pure Ti substrate by micro arc oxidation, Surf. Coat. Technol., 349(2018), 522–528, doi: 10.1016/j.surfcoat.2018.06.024
[14] F. Simchen; M. Sieber; T. Lampke: Electrolyte influence on ignition of plasma electrolytic oxidation processes on light metals. Surf. Coat. Technol. 315(2017), 205–213, doi: 10.1016/j.surfcoat.2017.02.041
[15] M. Kaseem; B. Dikici: Optimization of surface properties of plasma electrolytic oxidation coating by organic additives: A review, Coatings, 11(2021)4, 374, doi: 10.3390/coatings11040374. www.mdpi.com/2079-6412/11/4/374/htm[16]G.E. Thompson; F. Monfort; E. Matykina; A. Berkani; P. Skeldon: Coating generation by spark anodizing of light alloy, Corros. Rev., 25(2007)5-6, 631–650, doi: 10.1515/corrrev.2007.25.5-6.631
[17] Z. Yao; B. Hu; Q. Shen; A. Niua; Z. Jiang; P. Su; P. Ju: Preparation of black high absorbance and high emissivity thermal control coating on Ti alloy by plasma electrolytic oxidation, Surf. Coat. Technol., 253(2014), 166–170, doi: 10.1016/j.surfcoat.2014.05.032
[18] Z. Yao; X. Li; H. Wei; Q. Xia; Y. Wang; D. Li; Z. Jiang: Black ceramic coatings on Ti alloy with enhanced high absorptivity and high emissivity by plasma electrolytic oxidation, Int. J. Appl. Ceram. Technol., 16(2019)3, 994–1003, doi: 10.1111/ijac.13198
[19] R. Yao; Y. Li; Z. Yao; P. Zhang; S. Lu; X. Wu: Black PEO coating with enhanced thermal stability on titanium alloy and its thermal control properties, Surf. Coat. Technol., 429(2022), 127934, doi: 10.1016/j.surfcoat.2021.127934
[20] S. Stojadinovic; N. Tadic; N. Radic; B. Grbic; R. Vasilic: Effect of Tb3+ doping on the photocatalytic activity of TiO2 coatings formed by plasma electrolytic oxidation of titanium, Surf. Coatings Technol., 337(2018), 279–289, doi: 10.1016/j.surfcoat.2018.01.033
[21] P. Huang; F. Wang; K.W. Xu; Y. Han: Mechanical properties of titania prepared by plasma electrolytic oxidation at different voltages, Surf. Coat. Technol., 201(2007)9-11, 5168–5171, doi: 10.1016/j.surfcoat.2006.07.137
[22] F. Liu; J.L. Xu; D.Z. Yu; F.P. Wang; L.C. Zhao: Effects of cathodic voltages on the structure and properties of ceramic coatings formed on NiTi alloy by micro-arc oxidation, Mater. Chem. Phys., 121(2010)1-2, 172–177, doi: 10.1016/j.matchemphys.2010.01.002
[23] K. Rokosz; T. Hryniewicz; L. Dudek; K. Pietrzak; S. Raaen; W. Malorny; R Ciuperca: SEM, EDS and XPS studies of AC & DC PEO coatings obtained on titanium substrate, IOP Conf. Ser.: Mater. Sci. Eng., 564(2019), 012043, doi: 10.1088/1757-899X/564/1/012043
[24] F. Songur; B. Dikici; M. Niinomi; E. Arslan: The plasma electrolytic oxidation (PEO) coatings to enhance in-vitro corrosion resistance of Ti-29Nb-13Ta-4.6Zr alloys: The combined effect of duty cycle and the deposition frequency, Surf. Coat. Technol., 374(2019), 345–354, doi: 10.1016/j.surfcoat.2019.06.025
[25] S. Gowtham; T. Arunnellaiappan; N. Rameshbabu: An investigation on pulsed DC plasma electrolytic oxidation of cp-Ti and its corrosion behaviour in simulated body fluid, 2016 Surf. Coat. Technol., 301(2016), 63–73, doi: 10.1016/j.surfcoat.2016.02.043
[26] S. Lederer; S. Arat; W. Fuerbeth; Influence of process parameters on the tribological behavior of PEO coatings on CP-Titanium 4+ alloys for biomedical applications, Materials, 14(2021)18, 5364, doi: 10.3390/ma14185364
[27] H. Habazaki; S. Tsunekawa; E. Tsuji; T. Nakayama: Formation and characterization of wear-resistant PEO coatings formed on β-titanium alloy at different electrolyte temperatures, Appl. Surf. Sci., 259(2012), 711–718, doi: 10.1016/j.apsusc.2012.07.104
[28] S. Cheng; D. Wei; Y. Zhou: The effect of oxidation time on the micro-arc titanium dioxide surface coating containing Si,Ca and Na, Procedia Eng., 27(2012), 713–717, doi: 10.1016/j.proeng.2011.12.510
[29] X. Lu; C. Blawert; M.L. Zheludkevich; K.U. Kainer: Insights into plasma electrolytic oxidation treatment with particle addition, Corr. Sci., 101(2015), 201–207 doi: 10.1016/j.corsci.2015.09.016
[30] M. Mu; J. Liang; X. Zhou; Q. Xiao: One-step preparation of TiO2/MoS2 composite coating on Ti6Al4V alloy by plasma electrolytic oxidation and its tribological properties, Surf. Coat. Technol., 214(2013), 124–130, doi: 10.1016/j.surfcoat.2012.10.079
[31] M. Aliofkhazraei; A. Sabour Rouhaghdam; T. Shahrabi: Abrasive wear behaviour of Si3N4/TiO2 nanocomposite coat[31] M. Aliofkhazraei; A. Sabour Rouhaghdam; T. Shahrabi: Abrasive wear behaviour of Si3N4/TiO2 nanocomposite coatings fabricated by plasma electrolytic oxidation, Surf. Coat. Technol., 205(2010)Suppl.1, S41–S46, doi: 10.1016/j.surfcoat.2010.03.052
[32] K.R. Shin; Y.S. Kim; G.W. Kim; H.W. Yang; Y.G. Ko; D.H. Shin: Effects of concentration of Ag nanoparticles on surface structure and in vitro biological responses of oxide layer on pure titanium via plasma electrolytic oxidation, Appl. Surf. Sci., 347(2015) 574–582, doi: 10.1016/j.apsusc.2015.04.161
[33] M. Aliofkhazraei; R.S. Gharabagh; M. Teimouri; M. Ahmadzadeh; G.B. Darband; H. Hasannejad: Ceria embedded nanocomposite coating fabricated by plasma electrolytic oxidation on titanium, J. Alloys Compds, 685(2016), 376-383. doi: 10.1016/j.jallcom.2016.05.315
[34] P. Molaeipour; S.R. Allahkaram; S. Akbarzadeh: Corrosion inhibition of Ti6Al4V alloy by a protective plasma electrolytic oxidation coating modified with boron carbide nanoparticles, Surf. Coat. Technol., 430(2022), 127987, doi: 10.1016/j.surfcoat.2021.127987
[35] X. Lu; M. Mohedano; C. Blawert; E. Matykina; R. Arrabal; K.U. Kainer; M.L. Zheludkevich: Plasma electrolytic oxidation coatings with particle additions - A review, Surf. Coat. Technol., 307(2016), 1165–1182, doi: 10.1016/j.surfcoat.2016.08.055
[36] A. Lugovskoy; S. Lugovskoy: Production of hydroxyapatite layers on the plasma electrolytically oxidized surface of titanium alloys, Mater. Sci. Eng. C, 43(2014), 527–532, doi: 10.1016/j.msec.2014.07.030
[37] J. Karbowniczek; F. Muha; G. Cempura; H. Cimenoglu; A. Czyrska-Filemonowicz: Influence of electrolyte composition on microstructure, adhesion and bioactivity of micro-arc oxidation coatings produced on biomedical Ti6Al7Nb alloy, Surf. Coat. Technol., 321(2017), 97–107, doi: 10.1016/j.surfcoat.2017.04.031
[38] F. Liu; F. Wang; T. Shimizu; K. Igarashi; L. Zhao: Formation of hydroxyapatite on Ti-6Al-4V alloy by microarc oxidation and hydrothermal treatment. Surf. Coat. Technol., 199(2005), 220–224, doi: 10.1016/j.surfcoat.2004.10.146
[39] W. Zhang; K. Du; C. Yan; F. Wang: Preparation and characterization of a novel Si-incorporated ceramic film on pure titanium by plasma electrolytic oxidation, Appl. Surf. Sci., 254(2008)16, 5216–5223, doi: 10.1016/j.apsusc.2008.02.047
[40] M. Montazeri; C. Dehghanian; M. Shokouhfar; A. Baradaran: Investigation of the voltage and time effects on the formation of hydroxyapatite-containing titania prepared by plasma electrolytic oxidation on Ti-6Al-4V alloy and its corrosion behavior, Appl. Surf. Sci., 257(2011)16, 7268–7275, doi: 10.1016/j.apsusc.2011.03.103
[41] A. Kossenko; S. Lugovskoy; N. Astashina; A. Lugovskoy;
M. Zinigrad: Effect of time on the formation of hydroxyapatite in PEO process with hydrothermal treatment of the Ti-6Al-4V Alloy, Glass Phys. Chem., 39(2013)6, 639–642, doi: 10.1134/S1087659613060072
[42] Y.-K. Lee; H. Won; K. Roa, Y.-C. Ha; K.-H. Koo: Bipolar hemiarthroplasty using microarc oxidation-coated cementless stem in patients with unstable intertrochanteric fracture. J. Orthop. Surg. (Hong Kong), vol. 27(2019), 1–7, doi: 10.1177/2309499019847815.
[43] E. Parfenov; L. Parfenova; V. Mukaeva; R. Farrakhov; A. Stotskiy; A. Raab; K. Danilko; N. Rameshbabu; R. Valiev: Biofunctionalization of PEO coatings on titanium implants with inorganic and organic substances, Surf. Coat. Technol., 404(2020), 126486, doi: 10.1016/j.surfcoat.2020.126486
[44] S.-C. Liao; C.-T. Chang; C.-Y. Chen; C.-H. Lee; W.-L. Lin: Functionalization of pure titanium MAO coatings by surface modifications for biomedical applications, Surf. Coat. Technol., 394(2020), 125812, doi: 10.1016/j.surfcoat.2020.125812
[45] Creative Commons open access Licence, https://doi.org/10.1016/j.apsadv.2021.100121