Magnesium is an alkaline earth metal of group IIA having atomic number 12, atomic mass 24.31, and oxidation number +2. It is a silvery-white shiny metal similar in appearance to aluminium but weighs one-third less. With a density of only 1.738 g/cm3, it is the lightest structural metal known. In its pure form, magnesium lacks sufficient strength. Alloying with some other elements improves the strength and other properties of both cast and wrought magnesium alloys and make them suitable for wider applications. A designer has to take many factors into consideration while selecting the magnesium alloys for specific applications, viz., alloy composition, properties, forms of processing and finishing requirement, etc. [1].
History
Magnesium derives its name from Magnesia, a district in the ancient Greek region of Thessaly, where the deposits of magnesite, a magnesium carbonate mineral, was first found. The British chemist Sir Humphry Davy formed an amalgam of magnesium in 1808 by electrolyzing moist magnesium sulfate, using mercury as a cathode. The first metallic magnesium was produced in 1828 by the French scientist Antoine Bussy by the reduction of molten magnesium chloride with metallic potassium. The maiden industrial manufacturing of magnesium began in Germany in 1886 by Aluminum und Magnesiumfabrik Hemelingen, by electrolysis of molten carnallite. Later Hemelingen became part of the industrial complex IG Farbenindustrie, which is now branded as the IG Farben process [2].
Advantages and significance
Magnesium has many advantages and significance in diversified sectors:
- The lightest structural metal magnesium is 33 % lighter than aluminium, 50 % lighter than titanium, and 75 % lighter than steel
- High strength-to-weight ratio
- Provides very effective electromagnetic interference shielding without filters
- Excellent castability to net shape, better than aluminium
- High damping capacity, impact resistance, and pressure tightness
- Good thermal conductivity. Thin-wall components can be optimized with effective thermal management
- Good machinability, extremely fine and smooth surface finish can be achieved. Power requirements are 45 % less than aluminium
- 100 % recyclable, has one of the lowest carbon footprints of any structural material
- Biomaterial of high biological significance.
Biological systems: Magnesium is an essential element in biological systems. The eleventh most abundant element by mass in the human body, magnesium plays crucial roles in supporting the growth and metabolism to cells. ATP (adenosine triphosphate), the main source of energy in cells, is bound to a magnesium ion. Magnesium contributes to the catalytic functioning of over 300 enzymes. In plants, it is in the centre of the porphyrin ring of chlorophyll, the pigment responsible for the photosynthesis, without which life would not exist. Magnesium salts are included in many foods for animals and fertilizers for plants. Magnesium rich foods include legumes, nuts, spinach, cocoa, avocado, banana, nuts, pumpkins seeds, sprouts, and black beans. The daily requirement of magnesium to our body is 300 to 400 milligrams [3].
Therapeutic agent: Many magnesium compounds have therapeutic importance [4]:
The hydrous magnesium sulfate or Epsom salts, is used as a laxative, bath salts, and a highly soluble fertilizer
- Magnesium hydroxide suspended in water or milk of magnesia, is used as an antacid
- Magnesium hydroxide, chloride, glycinate, ascorbate, citrate, etc. are used as oral magnesium supplements.
- Magnesium borate, salicylate, and sulfate are used as antiseptics
- Magnesium carbonate powder is used by gymnasts, weightlifters, and climbers to eliminate palm sweat, and improve the grip
- Magnesium stearate has good lubricating properties, it is used in pharmaceutical manufacture to prevent sticking of tablets with the equipment while compressing
- Magnesium acetylsalicylate is used as a painkiller.
Biomedical importance: An important application of magnesium is in manufacturing of cardiovascular stents and orthopaedic biodegradable “smart” implants. Magnesium is a lightweight, biocompatible, and bioabsorbable metal with elastic modulus (41-45 GPa) closer to that of natural bone (3-20 GPa). This reduces the stress shielding of the bone around the implant [5-8].
End uses of magnesium
Magnesium alloys can be used in a variety of applications both structural and non-structural. The chart in Figure 1 shows the breakdown for annual consumption of magnesium metal [9] and its applications in different sectors.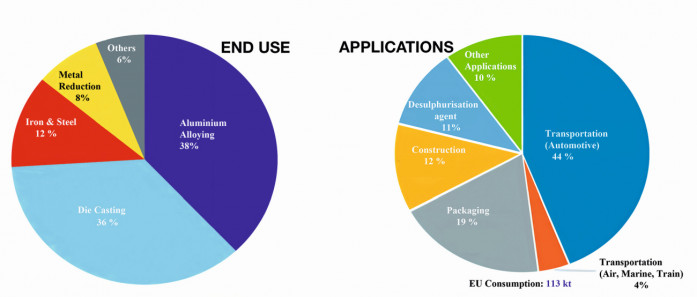 Fig.1: Global magnesium consumption/ Graphics: https://amt.co.uk & https://op.europa.eu/en/publication
Fig.1: Global magnesium consumption/ Graphics: https://amt.co.uk & https://op.europa.eu/en/publication
Metallurgical applications: By far magnesium is the most widely used alloying element in aluminium. Its amount ranges from 1-10 %, on average aluminium alloys contain about 1.5 % magnesium (by weight). Magnesium enhances the mechanical properties as well as the corrosion resistance of aluminium alloys. In fact, around 75 % of all finished products made from primary aluminium wouldn’t be suitable for their applications, if not contain magnesium as a critical alloying element, for which there is no substitute. Similarly, pure aluminium is used as an important alloying element in many magnesium-based alloys.
In the iron and steel industry, small quantity of magnesium is added to white cast iron to transform graphite into spherical nodules, thereby significantly improving the strength and malleability of the iron (called spheroidal or nodular cast iron). In addition, particulate magnesium blended with lime or other fillers is injected into liquid blast-furnace iron, where it improves mechanical properties of steel by combining with sulfur and oxygen. This is due to the strength of the crystal structure of magnesium, its high melting point, and its ability to remove sulfur from steel. The primary magnesium compound used in the refractory sector is dead burned magnesium (DBM), also referred to as refractory magnesia. Other metallurgical applications include the production of Ti, Zr, Hf, and U. The most significant of these is the Kroll process for reducing titanium tetrachloride to titanium metal by reaction with more reactive magnesium at high temperatures (800-900 °C) [9,10].
Alkaline refractory materials: A predominant industrial application of magnesium oxide compounds is in making refractory bricks. Magnesia bricks are made of magnesia sand, which are press-moulded, and electro fused or sintered at high temperature. Magnesia bricks are widely used in refractory furnaces for steel production, non-ferrous metallurgy, high temperature calcination, vertical, and tunnel furnaces. High purity magnesia bricks with a magnesium oxide content of more than 90 % and magnesite as the main crystalline phase are exceptionally wear- and temperature-resistant, with high heat capacity and conductivity. The expensive fused magnesia is utilized as insulating material in electric stoves and ovens, while the less expensive caustic magnesia is served in leaching lyes of paper industry.
Structural applications: Magnesium is the third-most-commonly-used structural metal, after iron and aluminium [11]. Comparison of important properties of Al, Mg, Ti, and steel are given in Table 1.
|
Property |
Magnesium / |
Aluminium / |
Titanium / |
Iron / |
|
Atomic number |
12 |
13 |
22 |
26 |
|
Atomic weight |
24.32 |
26.98 |
47.87 |
58.7 |
|
Melting point °C |
650 |
660 |
1668 |
1536 |
|
Boiling point °C |
1105 |
2520 |
3260 |
2862 |
|
Crystal structure |
HCP |
FCC |
HCP(α), BCC(β) |
BCC |
|
Elongation (%) |
6 (AZ91) |
12 (6061) |
10 (Ti6Al4V) |
20 (1010) |
|
Density at 20°C (g/cm3) |
1.74 1.81 (AZ91) |
2.70 2.70 (6061) |
4.51 4.43 (Ti6Al4V) |
7.87 |
|
Elastic modulus (GPa) |
45 (AZ91) |
70 (6061) |
114(Ti6Al4V) |
190–210 |
|
Yield Strength (MPa) |
160 (AZ91) |
275 (6061) |
1100 (Ti6Al4V) |
285–305 |
|
Poisson’s ratio |
0.35 |
0.33 |
0.32 |
0.30 |
|
Fatigue Strength (MPa) |
125–155 (AZ91) |
96.5 (6061) |
200–250 (Ti6Al4V) |
170–185 |
|
Electrical resistivity |
43.9 (AZ91) |
24.0 (6061) |
420 |
150 |
|
Thermal conductivity W/(m•K) |
72 (AZ91) |
167 (6061) |
11.4 6.7 (Ti6Al4V) |
50-65 |
|
Coefficient of thermal expansion |
26.0 (AZ91) |
23.6 (6061) |
9.0 (Ti6Al4V) |
12-14 |
|
Ultimate Tensile strength (MPa) |
240 (AZ91) |
290 (6061) |
860-1170 (Ti6Al4V) |
340-365 |
The mechanical properties of magnesium are improved by alloying with small amounts of other designated metals. The alloying elements form intermetallic compounds that permit heat treatment for enhanced mechanical properties. Magnesium alloys can be divided into two types. The general-purpose alloys, suitable for applications up to 150 °C, contain 3.0-9.0 % Al, 0.5-3.0 % Zn, and about 0.2 % Mn. Special alloys that can be used up to 250 °C contain various amounts of Zn, Zr, Th, Ag, and Y and other rare-earth metals. In addition, high-purity magnesium alloys, with controlled low contents of Fe, Ni, and Cu, have good corrosion resistance. Some of the examples of structural applications of magnesium alloys in various sectors are illustrated in a Figure 2, these include: Fig.2: Structural applications of magnesium alloys/ Graphics: https://amt.co.uk
Fig.2: Structural applications of magnesium alloys/ Graphics: https://amt.co.uk
Aerospace/Defence: Magnesium alloys are used to fabricate aircraft, helicopter, missile, rocket, and spacecraft parts. RZ5, ZRE1, and EQ21 alloys have been the favoured material for helicopter transmission, aviation engine, and gearbox casings. However, for superior corrosion resistance, and high-temperature qualities of large castings of WE43/WE43 alloys have been used on the Euro copter-EC120, NH90 helicopters, and Sikorsky S92. AZ91D alloy is used in the majority of consumer drone frames, such as DJI Phantom and Mavic.
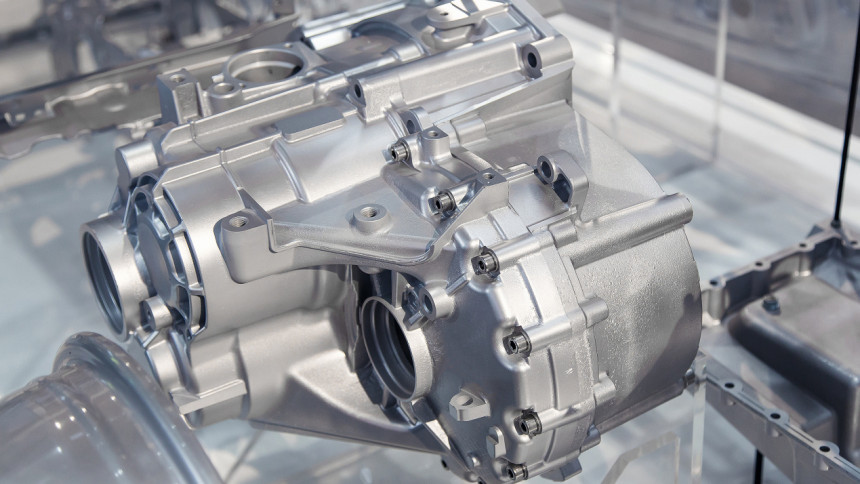
Automotive: The lightness of the material has a direct impact on the fuel efficiency of a vehicle, a 10 % reduction in the weight of a vehicle equals a 7 % improvement in fuel economy. Mg-Al-Mn-Ce, Mg-Al-Zn-Mn and Mg-Zn-Zr- alloys are preferred materials in the transport industry [8]. The automotive parts made out of magnesium alloys include: car wheels and seats, housings, transmission cases, engine blocks, support brackets for brakes and clutch, steering wheels and seat frames.
Electronics: Magnesium alloys are being used in computer housings, electronic packaging, hard drive arms, cell phone and portable media device housings, instead of plastics due to their light weight, strength and durability. They are also relatively better for heat dissipation and protection against electromagnetic and radio frequency interference [12].
Commercial: Al-Mg die-castings are used for manufacturing of beverage cans, sports equipment, laptops, camera bodies, etc. Zn-Mg sheets are used in making photoengraving plates in the printing industry, dry-cell battery walls, and roofing [13]. Other items include: bicycle frames, equipment for material handling, high-speed operating machinery, such as printing and textile machines, portable power tools, luggage, hand tools, ladders, portable medical equipment, wheelchairs.
Electrochemical applications: Magnesium is used in manufacturing primary rechargeable dry-cell batteries, and as a sacrificial (galvanic) anode in the cathodic protection of steel to resist corrosion of ships, boats, buried structures, water heaters, storage tanks, bridge structures, pipelines, and a variety of other steel products.
Chemical compounds: Being a strong reactant magnesium forms stable compounds naturally and synthetically which have wide commercial application:
- Dead Burned Magnesium (DBM) and Fused Magnesium (FM) in refractories
- Hard Burned Magnesium (HBM) in interior building products, fiberglass, and agriculture
- Light Burned Magnesium (LBM) in agriculture
- Dolomitic Lime in steel and glass making industries
- Caustic Calcined Magnesia (CCM) in water treatment fertilizers, animal nutrition products
- Magnesium di hydroxide (MDH) as fire retardants, fuel additives, and water treatment
- Magnesium hydride to store hydrogen
- Magnesium phosphate in fireproof wood
- Magnesium hexafluorosilicate for making moth-proof textiles
- Magnesium sulfate as a mordant for dyes
- Magnesium bisulfite is extensively used in the in the wood-pulping process for production of paper (sulfite process)
- Agricultural applications include the use of dolomite as a fertilizer in acidic soil and magnesium oxide as a mineral addition to cattle feed at the start of the grazing season in early spring
- Magnesium turnings react with an alkyl halide to prepare Grignard reagents, which is a very useful reagent for organic synthesis, e.g., preparing alcohols
Pyrotechnics: Magnesium reacts with oxygen at above 645 °C, burning with a bright white light and intense heat [14]. Magnesium powders are used to ignite pyrotechnics, incendiary bombs, and thermite devices that require a high ignition temperature. The finely divided particles of magnesium are used as a fuel component in conventional solid rocket propellants. Magnesium powders are used in flash photography, flares, fireworks, sparklers.
Drawbacks and mitigation: Despite sounding like a designers' “dream metal”, magnesium alloys have few drawbacks that limit their widespread applications: magnesium is about 10 % more expensive than aluminium, and it corrodes easily. Although its tensile yield strength is about the same, magnesium has lower ultimate tensile strength, fatigue strength, and creep strength compared to aluminium. The modulus and hardness of magnesium alloys is lower than aluminium and the thermal expansion coefficient is higher. However, many of these limitations can often be mitigated with suitable ribbing and support.
Part 2 will deal with the resources and production of magnesium
REFERENCES:
[1] Trang, T.T.T; Zhang, J.H.; Kim, J.H.; Zargaran, A.; Hwang, J.H.; Suh, B.-C.; Kim, N.J.: Designing a magnesium alloy with high strength and high formability, Nat. Commun. 9, no. 1 (2018) 2522. doi: 10.1038/s41467-018-04981-4
[2] Rizley, J.H.; Høy-Petersen, N.: Magnesium Processing, Encyclopedia Britannica, 19 Feb. 2020, https://www.britannica.com/technology/magnesium-processing, Accessed on 2023.01.31
[3] Magnesium in Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride, Washington (DC): National Academies Press, (1997) 190-249. doi: 10.17226/5776
[4] Magnesium, https://en.wikipedia.org/wiki/Magnesium, Accessed on 2023.01.31
[5] Chen Y.; Xu Z.; Smith, C.; Sankar J.: Recent advances on the development of magnesium alloys for biodegradable implants, Acta Biomater., 10, no. 11, (2014) 4561-4573. doi: 10.1016/j.actbio.2014.07.005
[6] Radha, R.; Sreekanth, D.: Insight of magnesium alloys and composites for orthopedic implant applications-A review, J. Magnes. Alloy., 5, no. 3 (2017) 286-312. doi: 10.1016/j.jma.2017.08.003
[7] Mallick, P.K.: Chapter 1 – Overview, Materials, Design and Manufacturing for Lightweight Vehicles, A volume in Woodhead Publishing in Materials, Second Edition, (2020) 1-36
[8] Predko,P.; Rajnovic D.; Grilli, M.L.; Postolnyi B.O.; Zemcenkovs V.; Rijkuris, G.; Pole, E.; Lisnanskis, M.: Promising methods for corrosion protection of magnesium alloys in the case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A recent progress review, Metals, 11, no. 7 (2021) 1133. doi: 10.3390/met11071133
[9] Amundsen, K.; Aune, T.K.;Bakke, P.; Eklund, H.R.; Haagensen, J.O.; Nicolas, C.; Rosenkilde, C.; den Bremt, S.V.; Wallevik, O.: Magnesium Ullmann's Encyclopaedia of Industrial Chemistry, Wiley-VCH, (2002). doi: 10.1002/14356007.a15_559
[10] Platacis, E.;Kaldre, I.; Blumbergs, E.; Goldsteins, L.; Serga, V.: Titanium production by magnesium thermal reduction in the electroslag process, Sci. Rep., 9 (2019) 17566. doi: 10.1038/s41598-019-54112-2
[11] Segal, D.: Materials for the 21st Century, Oxford Univ. Press, (2017) 1-312.
[12] Magnesium Applications, International Magnesium Association, https://www.intlmag.org/page/mg_applications_ima, Accessed on 2023.01.31
[13] Baker, H.; M.M. Avedesian: ASM Specialty Handbook: Magnesium and Magnesium Alloys, Materials Park, OH, (1999) p. 4
[14] Dreizin, E.L.; Berman, C.H.; Vicenzi, E.P.: Condensed-phase modifications in magnesium particle combustion in air, combustion and flame, 122, no. 1-2 (2000) 30-42. doi: 10.1016/S0010-2180(00)00101-2