The anodic oxide coating consists of two layers, the inner thin and dense barrier layer, and the more structured thick porous top layer. Anodic oxidation of titanium has attracted considerable interest as a simple, economical and fast electrochemical technique of surface modification. The formation of titanium oxides by anodizing in electrolytes composed of bioactive elements, has been reported as a very promising method either to enhance cell functionalities or to improve the tribocorrosion performance of titanium surfaces [6–11]. Metal anodization has been broadly used in industry as a surface treatment technique to render materials with resistance against uncontrolled oxidation, abrasion, and corrosion. Although this technique has been developed for a long time, it was until the 1990s that researchers discovered that highly ordered nano porous structures can be achieved by properly tuning anodization conditions including electrolyte composition and concentration and temperature, as well as anodization voltage. In general, most of the valve metals (Al, Ti, Ta, and Nb) can be anodized into nano porous structures with well-controlled diameter, pitch, and depth.
Applications
Anodization on titanium is carried out for various applications:
- To provide decorative finishing. As anodization on titanium under different conditions results in a range of stable attractive colours, it is used to produce a decorative effect on the jewellery and artifacts to improve their aesthetics, and for marking and identifying the products [11–14].
- To protect the metal from atmospheric corrosion. Titanium is a reactive metal; it is rapidly attacked by atmospheric oxygen. Anodizing greatly improves the corrosion resistance of titanium by providing a uniform stable protective film on the surface [5, 12–14].
- To improve abrasion resistance. Titanium anodizing leads to an improvement in abrasion resistance of products, which is particularly important for aviation, military and tool industries [5, 12].
- To reduce the friction on sliding surfaces. As an anodic film is generally harder than bare metal, it helps in reducing friction on moving parts. In addition, an anodic coating, owing to its porous nature, improves lubricity by providing a base for retention of lubricants. As titanium has a severe tendency to gall, lack of lubricity poses serious problems in applications involving the contact of sliding surfaces in various forming applications [5, 12].
- A good base for paints, lubricants and adhesives. Owing to specific texture and porous nature, anodic coating imparts a good bonding characteristic for paints, lubricants, adhesives and a variety of such other materials [5, 12–14].
- To impart thermal control properties. Anodized titanium acts as an excellent thermal control surface as it provides stable optical properties to its components.
- Titanium oxide nanotubes (TNT) with high-specific surface area show photocatalytic, ion-exchange properties, which has potential applications in various fields, photocatalysis [15–26], electro chromic device [27–29], hydrogen generation [30, 31], solar cells [32–34], sensors [35–41], storage device [42, 43], wastewater treatment [44] and nanomedicine applications [45].
- To introduce biocompatibility in implants. Due to micro roughness anodized titanium surface has higher bone-to-implant contact than non-anodized implants. Titania nanotubes with a diameter between 15–30 nm have a better interaction with cells, and higher diameters (between 70–100 nm) provoke a higher rate of cell apoptosis. Because of that, resultant TiO2 nanotube structures are known to have an encouraging effect in adhesion, migration, proliferation and survival of cells [46–48].
Mechanism and Morphology
Titanium is relatively easy to anodize in any solution that is capable of carrying current.A variety of electrolytes from strongly acidic to strongly basic can be employed [49]. Anodizing in acidic, neutral, and mildly basic solutions produces a very thin (up to 200 nm) transparent oxide coating. Anodizing of titanium in strong alkaline solutions can produce coatings up to several microns [50] which can be used to prevent galling and increase corrosion resistance.When titanium is immersed in an electrolytic solution as an anode and current is drawn, a flux of electrons is generated between both the electrodes. The electrolyte begins to decompose, the hydrogen ions move to the cathode where they get reduced to hydrogen gas. The oxygen ions generated at the anodic surface combine with the reactive titanium surface and a compact oxide layer starts growing on substrate through oxidation reactions, along with field-driven ion diffusion. Schematic diagram of the anodizing cell is shown in Figure 1.
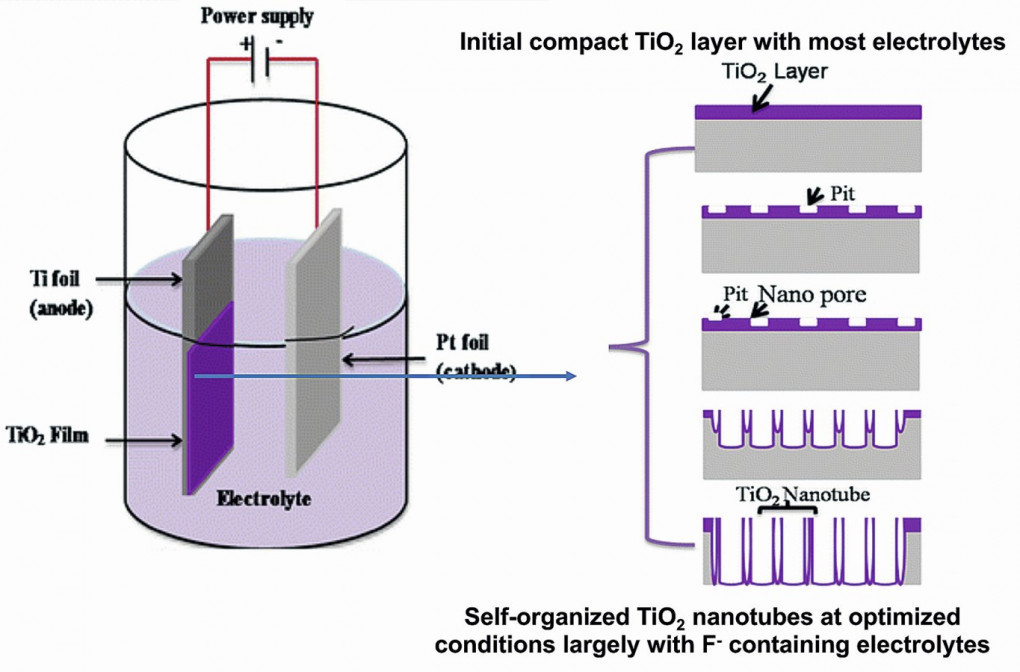 Fig. 1: Schematic diagram of anodizing cell with finished products [51,52]
Fig. 1: Schematic diagram of anodizing cell with finished products [51,52]
The chemical reactions of anodizing process in conventional sulphuric acid electrolyte can be expressed by the following equations:
Electrolytic reactions (ionization):
H2SO4 → 2H+ + SO42–
H2O → 2H+ + O2–
Anodic reaction:
At Ti/Ti oxide interface:
Ti → Ti2+ + 2e–
At Ti oxide/electrolyte interface:
2H2O → 2O2– + 4H+ (oxygen ions react with ti to form oxide)
2H2O → O2(g) + 4H+ + 4e– (oxygen gas evolves)
At both interfaces:
Ti2+ + 2O2– → TiO2 + 2e–
Cathodic reaction:
2H+ + 2 e– → H2 (g)
The presence of fluorides in the electrolyte strongly affects the anodization process, as fluorides form water-soluble [TiF6]2– species. At one hand complexation of ejected Ti4+ ions with fluoride at the oxide-electrolyte interface occurs:
Ti4+ + 6F– → [TiF6]2–
While on the other hand formed TiO2 dissolves as a soluble fluoride complex, which results in formation of self-organised nano porous structure:
TiO2 + 6F– + 4H2O → [TiF6]2– + 2H2O
Since the anodic titanium oxides have a higher resistivity to electrolyte than the substrate metal, the applied voltage will drop with the film growth. The reaction is self-limiting because as the oxide layer increases, its resistance to current rises, which eventually puts an end to the oxidation.
Coating Thickness and Colour
The oxide thickness is primarily controlled by operating voltage. However, other features of anodic titanium oxide film (morphology, topography, and chemical composition) are strongly influenced by processing conditions such as substrate material composition, pre-treatment, anodizing solution chemistry and temperature, load size, anode: cathode surface area ratio and distance, anodizing time, tank configuration, agitation speed of the solution etc. [5, 53]. There is almost a linear relationship between the final oxide thickness and the applied voltage (Figure 2).
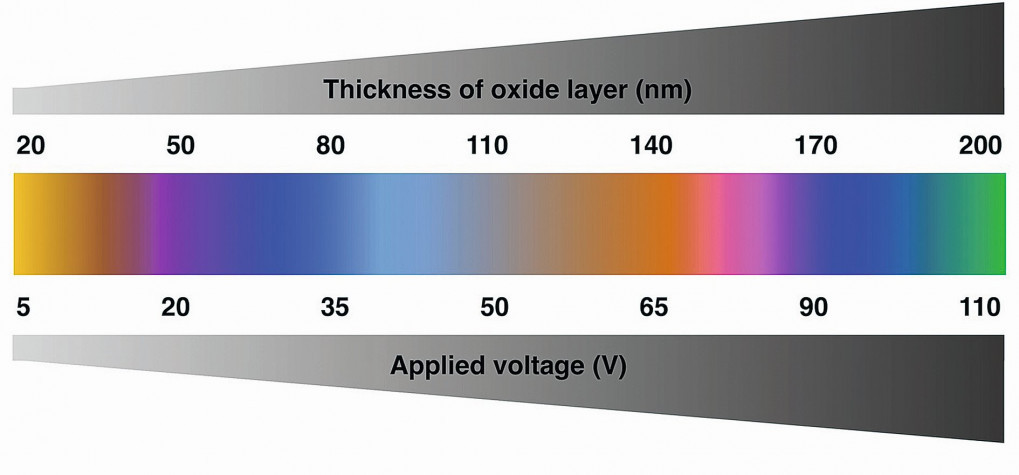 Fig. 2: Relationship between applied voltage and oxide layer thickness (Source: pressidium.com)
Fig. 2: Relationship between applied voltage and oxide layer thickness (Source: pressidium.com)
The oxides usually grow at the rate of 1.5–3.0 nm/V (also called as growth constant) in the various electrolytes [54]. However, this relationship holds well below the dielectric breakdown limit of the oxide, which is around 100 V depending on electrolyte and other process conditions [55]. At any given voltage the oxide film grows to a specific thickness and then stops when the resistance increases to a point where no current is being passed. The phenomenon of voltage-controlled oxide thickness indicates that the colour is also voltage controlled.
This thickness vs. resistance relationship dictates that the area of oxide produced by any specified voltage will not pass current at a lower voltage. In other words, an area that is anodized at 50 V need not be masked for anodizing adjacent areas at 30 V. It follows that multiple colour anodizing is to be carried out with decreasing voltage without masking the areas already anodized at higher voltages. Alternatively, the colours obtained at lower voltages (e.g., gold) can be changed into a higher voltage colour (e.g., blue) by re-applying a higher voltage that increases the thickness of the oxide layer.
The anodized parts have a vivid rainbow-like appearance due to interference colouring. The colours that are produced on titanium after anodizing are known as interference colours [16, 56, 57]. Depending on the thickness of the oxide, certain wavelengths (colours) will be in-phase and enhanced while other wavelengths will be out of phase and dampened. consequently, the colour is primarily determined by the oxide thickness. There are no pigments or dyes. Interference colours are produced by thin transparent films on a reflective surface. In nature, these colours can be seen in an oily, wet street and in the iridescent colours of some insects. The oxide film has the ability to reflect, refract and absorb light. White light falling on the oxide film is partly reflected and partly transmitted. The transmitted fraction reaching the metal surface is again partly absorbed but largely reflected back to oxide film. A phase shift occurs during this process together with multiple reflections. The degree of absorption and multiple reflection depends on the thickness of the film. The coloured light, i.e., light of restricted wavelength, results from the optical interference of light waves [58]. This phenomenon is represented in Figure 3. The oxide films grown on reactive metals (titanium, niobium and tantalum) in general have a higher refractive index than diamond. This accounts for the brilliance of the colour.
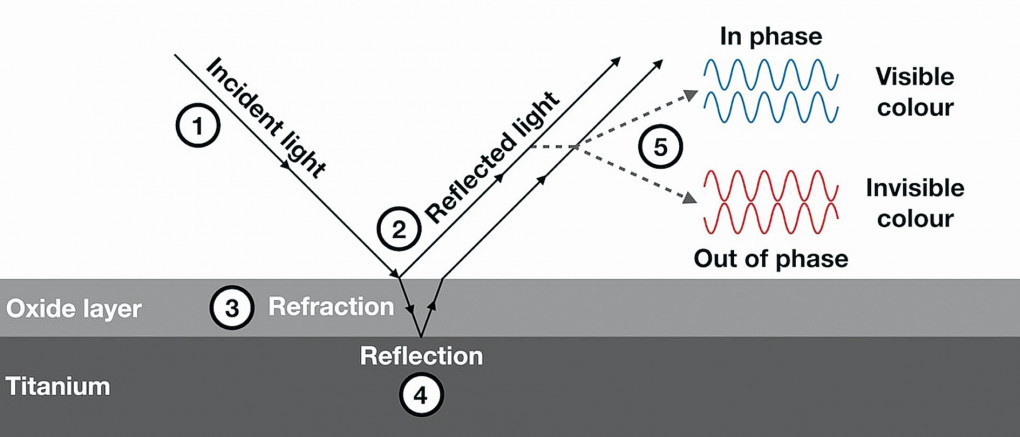 Fig. 3: The interference of light by titanium dioxide. When waves of light act on the surface of titanium (1),some are immediately reflected at the surface of the oxide layer (2). Others will be refracted (3)before they are reflected back to the surface (4) where they can interfere with one another (5). Those wavelengths (colours) that are in phase will be strengthened by this interference, while those that are out of phase will be cancelled out. The result is a filter of sorts that selects a specific colour, which is then perceived by the eye (Source: pressidium.com)
Fig. 3: The interference of light by titanium dioxide. When waves of light act on the surface of titanium (1),some are immediately reflected at the surface of the oxide layer (2). Others will be refracted (3)before they are reflected back to the surface (4) where they can interfere with one another (5). Those wavelengths (colours) that are in phase will be strengthened by this interference, while those that are out of phase will be cancelled out. The result is a filter of sorts that selects a specific colour, which is then perceived by the eye (Source: pressidium.com)
REFERENCES
[1]V.F. Henley: Anodic Oxidation of Aluminium and its Alloys, 1st Edition, Pergamon Press, Oxford, (1982)
[2]S. Wernick; R. Pinner: The Surface Treatment and Finishing of Aluminum and its Alloys, 4th Edition, Vol. 1&2, Robert Draper Ltd., Teddington, UK, (1972)
[3]A.K. Sharma: Conversion Coatings on Aluminium Alloys, Jahrbuch Oberflachentechnik, Eugen G. Leuze Verlag, 76(2020), 35-62
[4]Y. Lin; Q. Lin; X. Liu; Y. Gao; J. He; W. Wang; Z. Fan: A highly controllable electrochemical anodization process to fabricate porous anodic aluminum oxide membranes, Nanoscale Res. Lett., 10 (2015)1, 495. doi: 10.1186/s11671-015-1202-y
[5]A.K. Sharma: Anodizing titanium for space applications, Thin Solid Films, 208(1992)1, 48-54. doi: 10.1016/0040-6090(92)90946-9
[6]C. Yao; E.B. Slamovich; T.J. Webster: Enhanced osteoblast functions on anodized titanium with nanotube-like structures, J. Biomed. Mater. Res. A, 85(2008)1, 157-166. doi: 10.1002/jbm.a.31551
[7]N.A. El-wassefy; I.M. Hammouda; A.N.E.A. Habib; G.Y. El-awady; H.A. Marzook: Assessment of anodized titanium implants bioactivity, Clin. Oral Implants Res. 25(2014)2, e1-e9. doi: 10.1111/clr.12031
[8]H. Tang; Y. Li; J. Ma; X. Zhang; B. Li; S. Liu; F. Dai; X. Zhang: Improvement of biological and mechanical properties of titanium surface by anodic oxidation, Bio-Med. Mater. Eng. 27(2016)5, 485-494. doi: 10.3233/bme-161602
[9]S. Jain; R. Scott Williamson; M.D. Roach: Surface characterization, shear strength, and bioactivity of anodized titanium prepared in mixed-acid electrolytes, Surf. Coat. Technol., 325(2017), 594-603. doi: 10.1016/j.surfcoat.2017.07.010
[10]S.A. Alves; S.B. Patel; C. Sukotjo; M.T. Mathew; P.N. Filho; J.-P. Celis; L.A. Rocha; T. Shokuhfar: Synthesis of calcium-phosphorous doped TiO2 nanotubes by anodization and reverse polarization: A promising strategy for an efficient biofunctional implant surface, Appl. Surf. Sci., 399(2017), 682–701. doi: 10.1016/j.apsusc.2016.12.105
[11]M. Duvvuru; L. Wu; N. Lin; T. Xu; S. Vahabzadeh: Surface treatment of titanium by anodization and iron deposition: Mechanical and biological properties, J. Mater. Res., 35(2020)10, 1290-1297. doi: 10.1557/jmr.2020.107
[12]A.K. Sharma: Black Electrochemical Coatings for aerospace and allied Applications, Part 3- Black Anodic Oxide Coatings, Galvanotechnik, 112(2021)8, 1009-1016
[13]L. Young: Anodic Oxide Films, Academic Press, London; New York, (1961), 1–377
[14]Hel Badekas; Chr. Panagopoulos: Titanium anodization under constant voltage conditions, Surf. Coat. Technol., 31(1987)4, 381-388. doi: 10.1016/0257-8972(87)90164-2
[15]L.L. Costa; A.G.S. Prado: TiO2 nanotubes as recyclable catalyst for efficient photocatalytic degradation of indigo carmine dye, J. Photochem, Photobiol. A: Chem., 200(2009)1, 45–49. doi: 10.1016/j.jphotochem.2008.09.014
[16]H.C. Liang; X.Z. Li: Visible-induced photocatalytic reactivity of polymer-sensitized titania nanotube films, Appl. Catal. B, 86(2009)1-2, 8–17, doi: 10.1016/j.apcatb.2008.07.015
[17]T.-J. Whang; H.-Y Huang; M.-T Hsieh; J.-J. Chen: Laser-induced silver nanoparticles on TiO2 for photocatalytic degradation of methylene blue, Int. J. Mol. Sci., 10(2009)11, 4707-4718. doi: 10.3390/ijms10114707
[18]S. Liu; L. Yang; S. Xu; S. Luo; Q. Cai: Photocatalytic activities of C-N-doped TiO2 nanotube array/carbon nanorod composites, Electrochem. Commun., 11(2009)9, 1748-1751, doi: 10.1016/j.elecom.2009.07.007
[19]Y.S. Sohn; Y.R. Smith; M. Misra; V. (Ravi) Subramanian: lectrochemically assisted photocatalytic degradation of methyl orange using anodized titanium dioxide nanotubes, Appl Catal B, 84(2008)3-4, 372–378, doi: 10.1016/j.apcatb.2008.04.021
[20]L. Sun; J. Li; C.L. Wang; S.F. Li; H.B. Chen; C.J. Lin: An Electrochemical strategy of Doping Fe3+ into TiO2 nanotube array films for enhancement in photocatalytic activity, Sol. Energy Mater. Sol. Cells, 93(2009)10, 1875–1880, doi: 10.1016/j.solmat.2009.07.001
[21]Y.B. Xie; X.Z. Li: Preparation and characterisation of TiO2/ Ti film electrodes by anodization at low voltage for photocatalytic application, J Appl. Electrochem. 36(2006)6, 663–668, doi: 10.1007/s10800-006-9118-y
[22]H. Li; B. Zhu; Y. Feng; S. Wang; S. Zhang; W. Huang: Synthesis, characterisation of TiO2 nanotubes-supported MS (TiO2NTs@MS, M=Cd, Zn) and their photocatalytic activity, J Solid State Chem 180(2007)7, 2136–2142, doi: 10.1016/j.jssc.2007.05.013
[23]K.O. Awitor; S. Rafqah; G. Geranton; Y. Sibaud; P.R. Larson; R.S.P. Bokalawela; J.D. Jernigen; M.B. Johnson: Photo-catalysis using titanium dioxide nanotube layers, J. Photochem. Photobiol. A Chem, 199(2008)2–3, 250–254, doi: 10.1016/j.jphotochem.2008.05.023
[24]I. Paramasivam; A. Avhale; A. Inayat; A. Bosmann; P. Schmuki; W. Schwieger: MFI-type (ZSM-5) Zeolite filled TiO2 nanotubes for enhanced photocatalytic activity, Nanotechnology, 20(2009)22, 225607, doi: 10.1088/0957-4484/20/22/225607
[25]Z. Zhang; Y. Yuan; Y. Fang; L. Liang; H. Ding; G. Shi; L. Jin: Photoelectrochemical oxidation behavior of methanol on highly ordered TiO2 nanotube array electrodes, J. Electro-
anal. Chem., 610(2007)2, 179–185, doi: 10.1016/j.jelechem.2007.07.028
[26]J. Geng; Z. Jiang; Y. Wang; D. Yang: Carbon modified TiO2 nanotubes with enhanced photocatalytic activity synthesized by a facile wet chemistry method, Scr., Mater., 59(2008)3, 352–355, doi: 10.1016/j.scriptamat.2008.04.004
[27]J.M. Macak; H. Tsuchiya; A. Ghicov; K. Yasuda; R. Hahn; S. Bauer; P. Schmuki: TiO2 nanotubes: self-organised electrochemical formation, properties and applications, Curr, Opin, Solid State Mater, Sci., 11(2007)1-2, 3–18, doi: 10.1016/j.cossms.2007.08.004
[28]A. Ghicov; P. Schmuki: Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned mox structures, Chem. Commun., 20(2009), 2791–2802, doi: 10.1039/b822726h
[29]A. Ghicov; H. Tsuchiya; J.M. Hahn Macak; A.G. Munoz; P. Schmuki: TiO2 nanotubes: H+ insertion and strong electrochromic effects, Electrochem, Commun., 8(2006)4, 528–532, doi: 10.1016/j.elecom.2006.01.015
[30]Z. Liu; K.S. Raja; R.R. Rangaraju; M. Misra: Hydrogen generation under sunlight by self-ordered TiO2 nanotube arrays, Int. J. Hydrogen Energy, 34(2009)8, 3250–3257, doi: 10.1016/j.ijhydene.2009.02.044
[31]S. Bae; E. Shim; J. Yoon; H. Joo: Enzymatic hydrogen production by light-sensitized anodized tubular TiO2 photoanode, Sol. Energy Mater. Sol. Cells, 92(2008)4, 402–409, doi: 10.1016/j.solmat.2007.09.019
[32]G.K. Mor; O.K. Varghese; M. Paulose; A. Shankar; C.A. Grimes: A Review on highly ordered, vertically oriented TiO2 nanotube arrays: fabrication, material properties, and solar energy applications, Sol. Energy Mater. Solar Cells, 90(2006)14, 2011–2075, doi: 10.1016/j.solmat.2006.04.007
[33]N.K. Allam; C.A. Grimes: Room temperature one-step polyol synthesis of anatase TiO2 nanotube arrays: photoelectrochemical properties, Langmuir, 25(2009)13, 7234–7240, doi: 10.1021/la9012747
[34]X.D. Li; D.W. Zhang; Z. Sun; Y.W. Chen; S.M. Huang:Metal-free Indoline-dye sensitized TiO2 nanotube solar cells, Microelectron J., 40(2009)1, 108–114, doi: 10.1016/j.mejo.2008.06.045
[35]O.K. Varghese; D. Gong; M. Paulose; K.G. Ong; C.A. Grimes: Hydrogen sensing using titania nanotubes. Sensor. Actuat. B, 93(2008), 338–344, doi: 10.1016/S0925-4005(03)00222-3
[36]S. Banerjee; S.K. Mohapatra; M. Misra; I.B. Mishra: The detection of improvised nonmilitary peroxide based explosives using a titania nanotube array sensor, Nanotechnology, 20(2009)7, 075502, doi: 10.1088/0957-4484/20/7/075502
[37]M.H. Seo; M. Yuasa; T. Kida; J.-S. Huh; K. Shimanoe; N. Yamazoe: Gas sensing characteristics and porosity control of nanostructured films composed of TiO2 nanotubes, Sens Actuators B: Chem., 137(2009)2, 513–520, doi: 10.1016/j.snb.2009.01.057
[38]C. Guo; Hu F.; C.M. Li; P.K. Shen: Direct electrochemistry of hemoglobin on carbonized titania nanotubes and its application in a sensitive reagentless hydrogen peroxide biosensor, Biosens. Bioelectron., 24(2008)4, 819–824, doi: 10.1016/j.bios.2008.07.007
[39]A.K.M. Kafi; G. Wu; A. Chen: A novel hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase onto Au-modified titanium dioxide nanotube arrays, Biosens. Bioelectron., 24(2008)4, 566–571, doi: 10.1016/j.bios.2008.06.004
[40]Y. Xie; L. Zhou; H. Huang: Bioelectrocatalytic application of titania nanotube array for molecule detection, Biosens. Bioelectron., 22(2007)12, 2812–2818, doi: 10.1016/j.bios.2006.11.016
[41]R. Zhao; M. Xu; J. Wang; G. Chen: A pH sensor based on the TiO2 nanotube array modified Ti electrode, Electrochim. Acta, 55(2010)20, 5647–5651, doi: 10.1016/j.electacta.2010.04.102
[42]P. Pillai; K.S. Raja; M. Misra: Electrochemical storage of hydrogen in nanotubular TiO2 arrays, J. Power Sour., 16(2006)1, 524–530, doi: 10.1016/j.jpowsour.2006.03.088
[43]L.P. An; X.P. Gao; G.R. Li; T.Y. Yan; H.Y. Zhu; Shen P.W.: Electrochemical lithium storage of titania nanotube modified with NiO nanoparticles, Electrochim. Acta, 53(2008)3, 4573–4579, doi: 10.1016/j.electacta.2007.12.075
[44]J. Zhang; B. Zhou; Q. Zheng; J. Li; J. Bai; Y. Liu; W. Cai: Photocatalytic COD determination method using highly ordered TiO2 nanotube array, Water Res., 43(2009)7, 1986–1992,
doi: 10.1016/j.watres.2009.01.035
[45]M. Kulkarni; A. Mazare; P. Schmuki; A. Iglic: Chapter 5, Biomaterial surface modification of titanium and titanium alloys for medical applications in Nanomedicine, A. Seifalian; A. de Mel; D.M. Kalaska (Editors), One Central Press, (2014), 112–130
[46]J. Park; S. Bauer; K. Von der Mark; P. Schmuki: Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett., 7(2007)6, 1686–1691, doi: 10.1021/nl070678d
[47]I. Jang; S. Shim; D. Choi; B. Cha; J. Lee; B. Choe; B. Choi: Effect of TiO2 nanotubes arrays on osseointegration of orthodontic miniscrew. Biomed Microdevices, 17(2015), 76, doi: 10.1007/s10544-015-9986-1
[48]S. Yavari; Y. Chai; A. Bottger; R. Wauthle; J. Schrooteng; H. Weinans; A. Zadpoor: Effects of anodizing parameters and heat treatment on nanotopographical features, bioactivity, and cell culture response of additively manufactured porous titanium, Mater. Sci. Eng. C, 51(2015), 132–138,
doi: 10.1016/j.msec.2015.02.050
[49]B. Seeley: Anodizing the reactive metals, Met. Finish., 84(1986)8, 11–13
[50]Anodic Treatment-Titanium and Titanium Alloys Solution pH 13 or Higher, AMS2488E, 2019-10-31
[51]J.Z. Mbese; Madikizela Z.: Structural and morphological properties of TiO2 nanotubes fabricated via electro-anodization process, J. Material Sci. Eng., 8(2019)1, 1000509,doi: 10.4172/2169-0022.1000509
[52]J.V. Patil; S.S. Mali; C.K. Hong; J.H. Kim; P.S. Patil: Structural, morphological, and wettability study of electrochemically anodized 1D TiO2 nanotube arrays, Appl. Phys. A, 123(2017)10, 610, doi: 10.1007/s00339-017-1197-6
[53]D. Dunn; S. Raghavan: Formation and characterization of anodized layers on CP Ti and Ti-6Al-4V biomaterials, Surf. Coat. Tech., 50(1992)3, 223–232, doi: 10.1016/0257-8972(92)90005-u
[54] C. Larsson; P. Thomsen; B.O. Aronsson; M. Rodahl; J. Lausmaa; B. Kasemo; L.E. Ericson: Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses, Biomater., 17(1996)6, 605–616, doi: 10.1016/0142-9612(96)88711-4
[55]J. Lausmaa: Mechanical, Thermal, Chemical and Electrochemical Surface Treatment of Titanium in Titanium in Medicine, D.M. Brunette; P. Tengvall; M. Textor; P. Thomsen (Editors), Springer, Germany, (2001), 231–266
[56] U. Balaji; S.K. Pradhan: Titanium anodisation designed for surface colouration – Systemisation of parametric interaction using response surface methodology, Mater. Des., 139(2018), 409–418, doi: 10.1016/j.matdes.2017.11.026
[57] M. Mallaiah; R.K. Gupta: Surface engineering of titanium using anodization and plasma treatment, IOP Conf. Ser.: Mater. Sci. Eng., 943(2020), 012016, doi: 10.1088/1757-899X/943/1/012016
[58]J.B. Cotton; P.C.S. Hayfield: Decorative finishes on titanium, Trans. Inst. Met. Finish, 45(1967)1, 48–52, doi: 10.1080/00202967.1967.11870018